Preparation method of anionic polymer membrane with high chemical stability
A technology of anionic polymer and chemical stability, applied in the field of preparation of anionic polymer membrane, can solve problems such as development space limitation, and achieve the effects of good mechanical strength, good chemical stability and high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
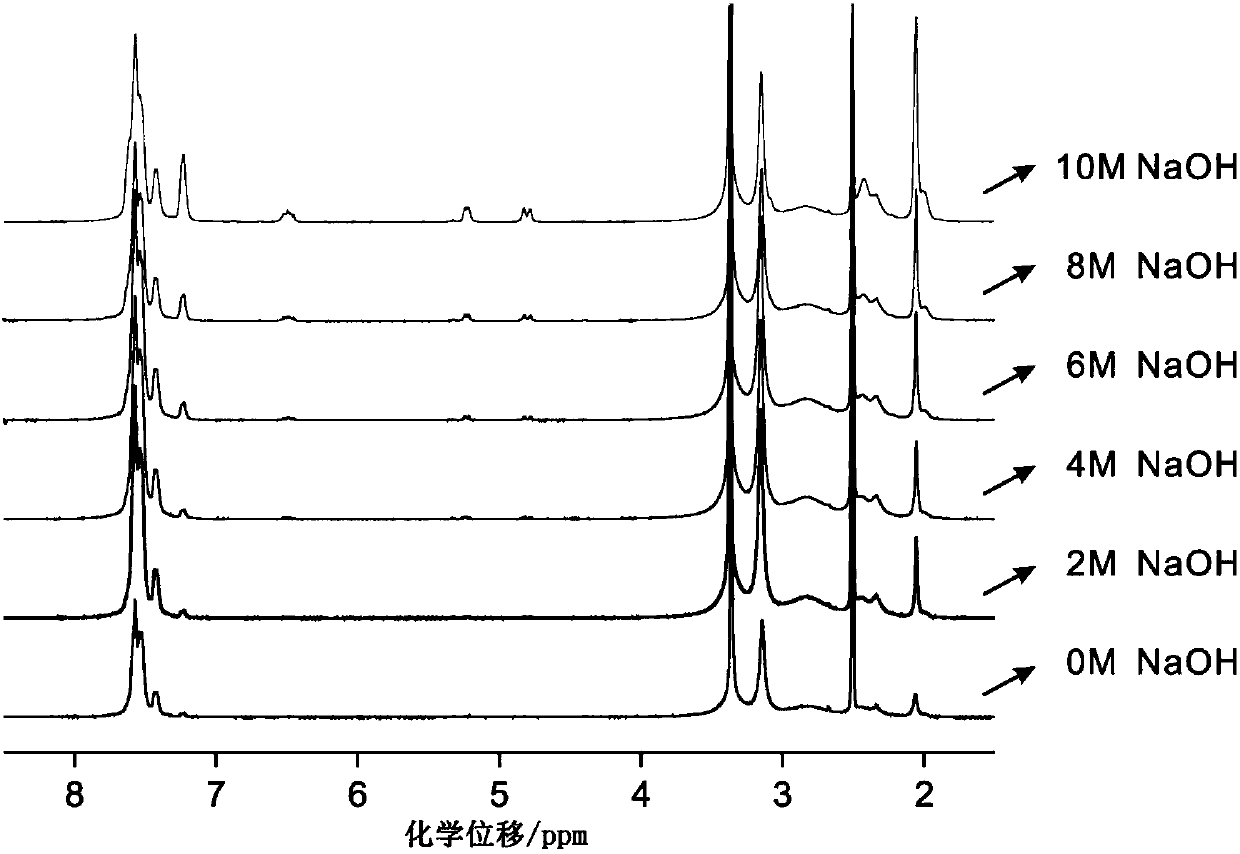
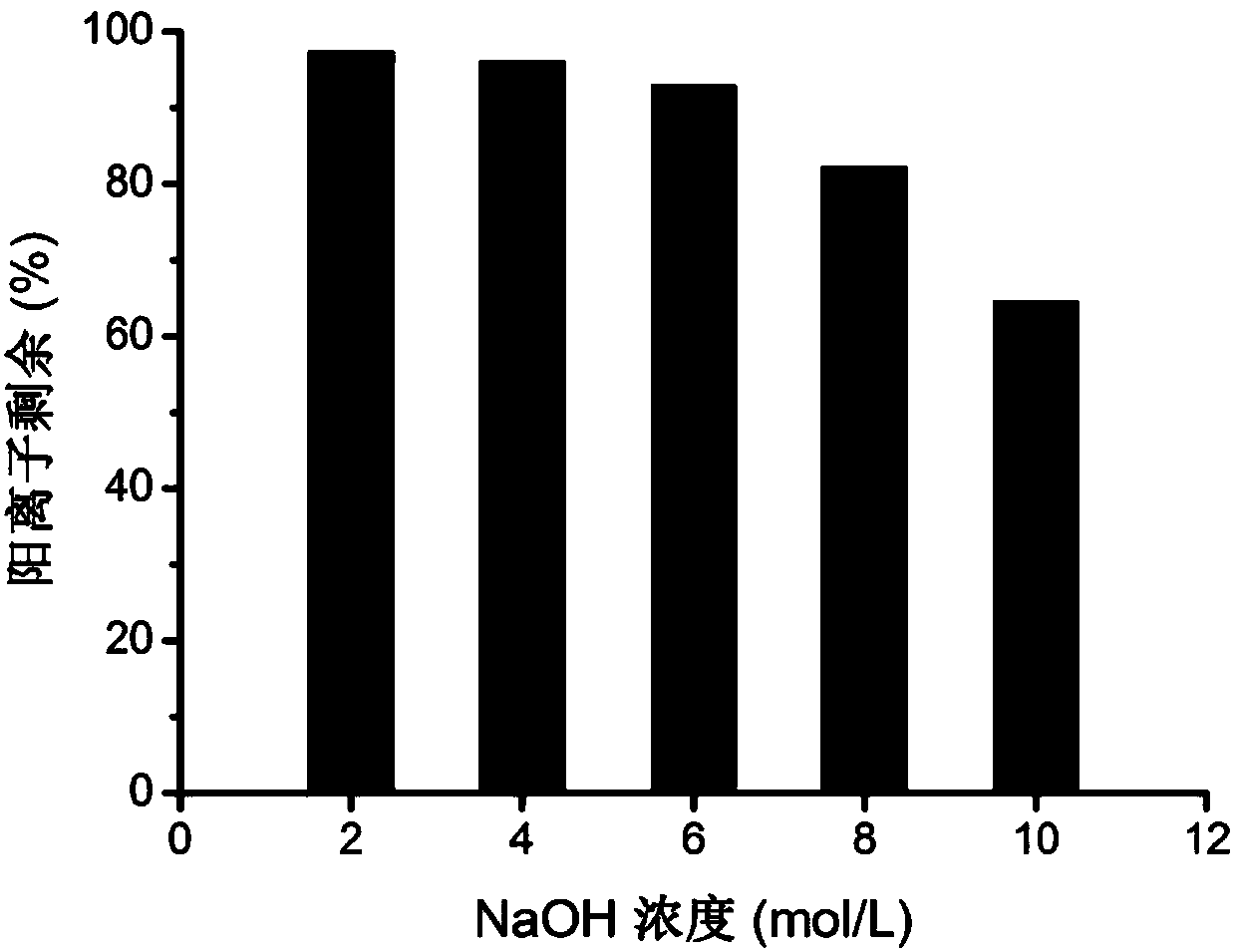
Image
Examples
Embodiment 1
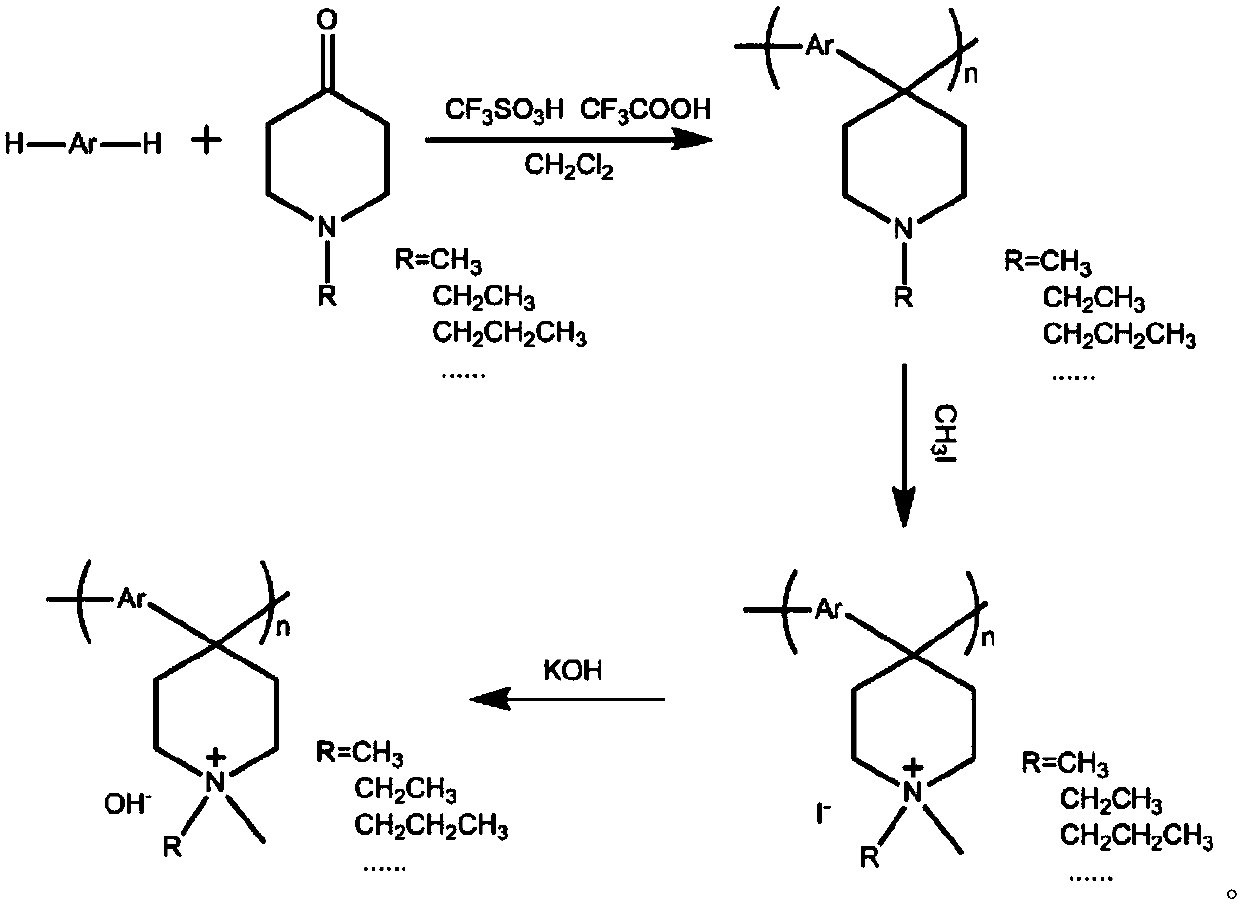
[0024] Weigh 41.0 mmol of biphenyl into a 150 mL three-necked flask, add 42.0 mmol of 1-methyl-4-piperidone, and add 8.6 mL of dichloromethane to dissolve the reactant. Under the condition of an ice-water bath, 32 mL of a mixed acid of trifluoromethanesulfonic acid and trifluoroacetic acid was added, the volume ratio of the two was 15:1, and the mixture was reacted for 3 hours. The product was precipitated in potassium carbonate solution, washed thoroughly with deionized water, and dried in an oven at 60-80°C for 24 hours. The initial polymer feed is obtained.
[0025] Weigh 10.0mmol of the above-mentioned polymer and add it into 1-methyl-2-pyrrolidone to dissolve it, and make a polymer solution with a mass fraction of 33.3-66.7 mg / mL, add 18.5-20 times the equivalent of methyl iodide at 40-60°C The reaction was carried out for 8-16 hours to obtain quaternized polymers with different reaction efficiencies. After the reaction was completed, it was precipitated in diethyl ethe...
Embodiment 2
[0031] The initial polymer raw material was synthesized according to the method of Example 1.
[0032] Weigh 10.0 mmol of the initial polymer raw material and add it to 1-methyl-2-pyrrolidone to dissolve it, and prepare a polymer solution with a mass fraction of 33.3-66.7 mg / mL, add 30-35 times the equivalent of ethyl bromide at 70- React at 75°C for 24 hours. After the reaction was completed, it was precipitated in diethyl ether to obtain a light white powdery solid, which was washed repeatedly with diethyl ether. A quaternized ionic polymer is obtained.
[0033]Soak the ionic polymer obtained above in 60°C 1mol / L KOH solution for 24 hours to fully exchange bromide ions into hydroxide ions. The solid was then centrifuged and washed several times until the supernatant was neutral. The resulting white solid was dried in an oven at 60-100° C. for 24-48 hours to obtain a white powdery solid. Dissolve the above solid in dimethyl sulfoxide, cast it on a glass plate, and dry it ...
Embodiment 3
[0036] Weigh 41.0 mmol of biphenyl into a 150 mL three-necked flask, add 43.0 mmol of 1-ethyl-4-piperidone, and add 8.0 mL of dichloromethane to dissolve the reactant. Under the condition of an ice-water bath, 32 mL of a mixed acid of trifluoromethanesulfonic acid and trifluoroacetic acid was added, with a volume ratio of 15:1, and reacted for 2 hours. The product was precipitated in potassium carbonate solution, washed thoroughly with deionized water, and dried in an oven at 60-80°C for 24 hours. The initial polymer feed is obtained.
[0037] Weigh 10.0mmol of the above-mentioned polymer and add it into 1-methyl-2-pyrrolidone to dissolve it to form a polymer solution with a mass fraction of 33.3-66.7 mg / mL, add 20-22 times the equivalent of methyl iodide and heat it at 70-75°C The reaction was carried out for 20-30 hours to obtain quaternized polymers with different reaction efficiencies. After the reaction was completed, it was precipitated in diethyl ether to obtain a yel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com