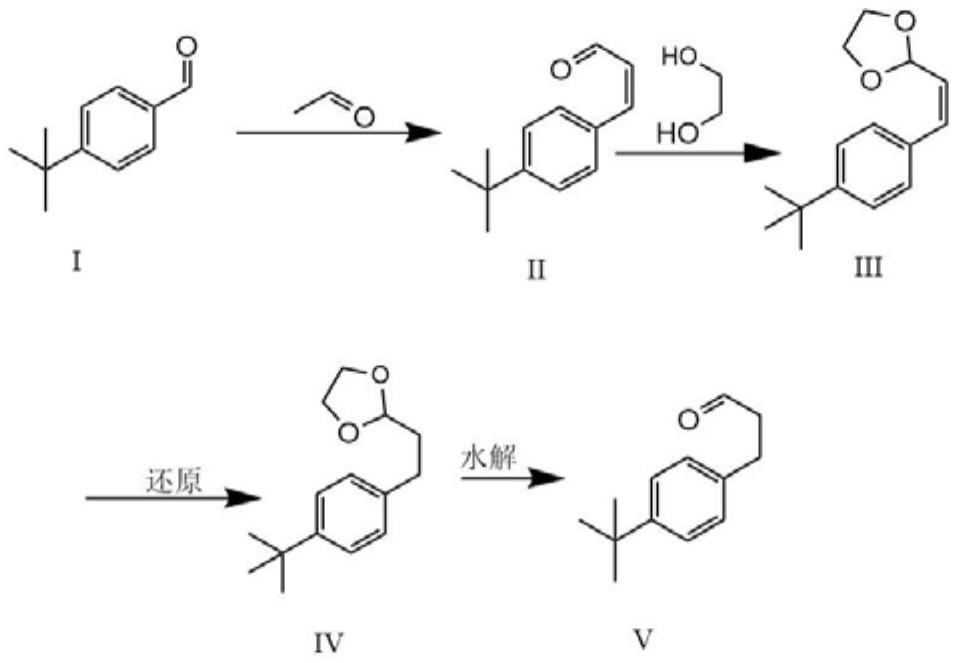
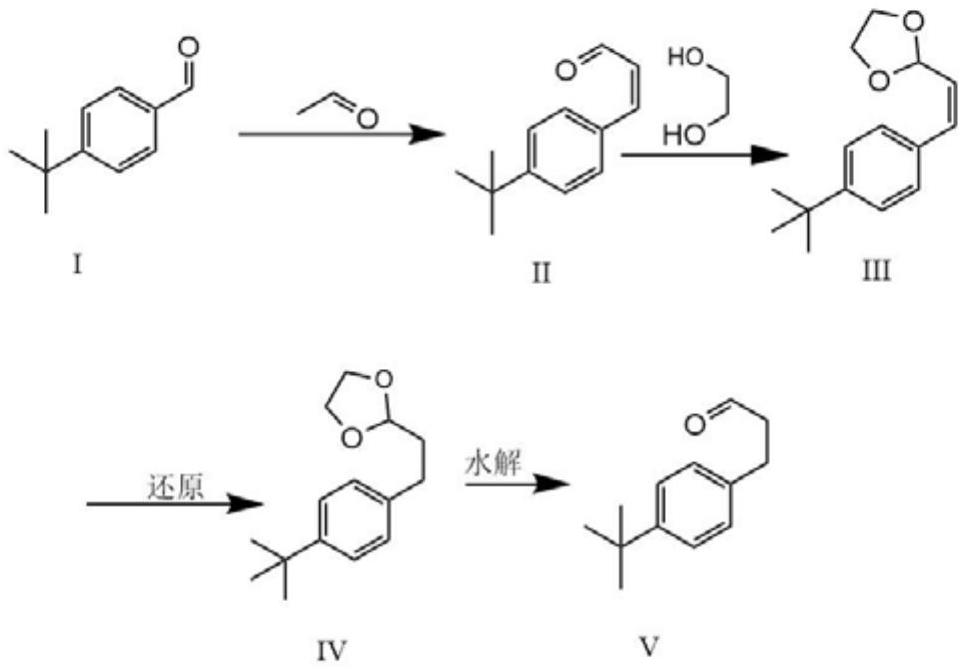
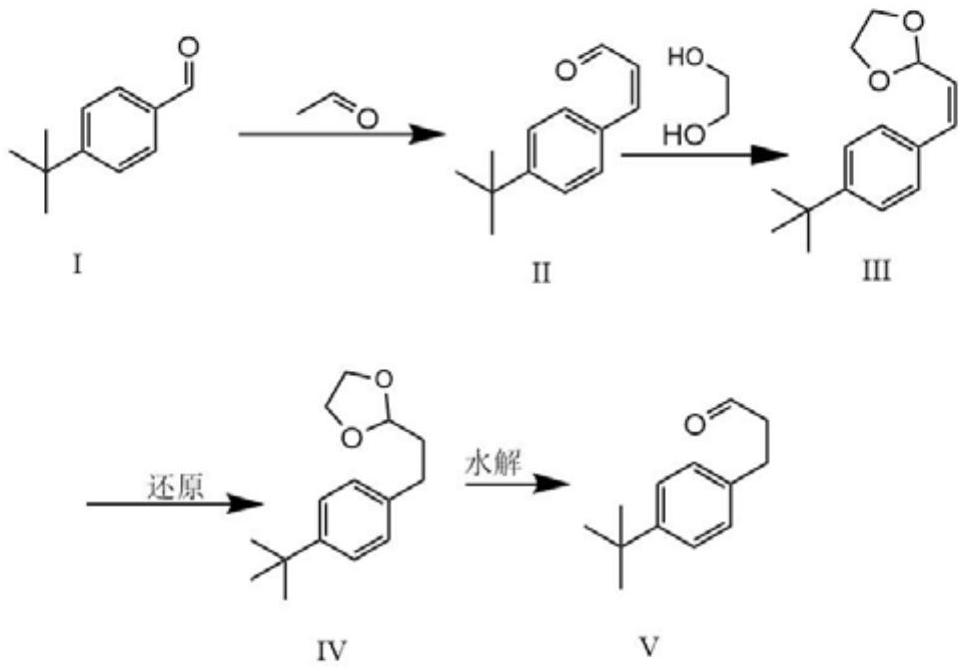
A kind of method of synthesizing p-tert-butylphenylpropionaldehyde
A technology for tert-butyl phenylpropionaldehyde and p-tert-butyl aldehyde, which is applied in the field of synthesizing p-tert-butyl phenylpropionaldehyde, can solve the problems of difficult suction filtration, high viscosity, difficult handling, etc., and achieves simple post-processing, little pollution, The effect of suction filtration with high viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Weigh 10g (0.062mol) of p-tert-butylbenzaldehyde and 3.26g (0.072mol) of acetaldehyde and dissolve it in toluene, then add 4g (0.1mol) of NaOH, reflux and distill off water, after the reaction is completed, adjust it with 10% hydrochloric acid PH = between 5 and 6, and then extracted to obtain 12 g of a mixture containing 3-(4-(tert-butyl)phenyl)acrylaldehyde. Dissolve 3-(4-(tert-butyl basic) phenyl) allyl aldehyde in toluene, ethylene glycol 20g and add 1g of p-toluenesulfonic acid under the catalysis, fractionate the water, after the reaction, wash the substrate with 5% Na 2 CO 3 The aqueous solution was washed 1-3 times, and then back-extracted once with ether, and the organic layers were combined to obtain 15 g of the mixture. Dissolve 15 g of the mixture with ethanol solution, add 2 g of palladium carbon, put it into a reaction bottle, replace the gas in the bottle with hydrogen, put a hydrogen balloon on it, and react overnight. After the reaction was complete, ...
Embodiment 2
[0019] Weigh 150g of p-tert-butylbenzaldehyde and 48.6g of acetaldehyde and dissolve it in 500ml of toluene, then add 45g (0.1mol) of NaOH, reflux and distill off water, after the reaction is completed, adjust the pH to between 5 and 6 with 10% hydrochloric acid , and then extracted to obtain 178 g of a mixture containing 3-(4-(tert-butyl)phenyl)acrylaldehyde. Dissolve 3-(4-(tert-butyl basic) phenyl) allyl aldehyde in toluene, 65 g of ethylene glycol and add 6 g of p-toluenesulfonic acid under the catalysis, and distill off water. After the reaction, the substrate is washed with 5% Na 2 CO 3 The aqueous solution was washed 1-3 times, and then back-extracted once with ether, and the organic layers were combined to obtain 175 g of the mixture. Dissolve 175g of the mixture with ethanol solution, add 15g of palladium carbon, put it into a reaction kettle, replace the gas in the bottle with hydrogen, pressurize to 3-7MPa, and react overnight. After the reaction was complete, the ...
Embodiment 3
[0021] Weigh 1.5kg of p-tert-butylbenzaldehyde and 611g of acetaldehyde and dissolve it in toluene, then add NaOH, reflux and distill off water, after the reaction is completed, use 10% hydrochloric acid to adjust the pH to between 5 and 6, and then extract to obtain 1.68 Kg contains 3-(4-(tert-butyl)phenyl)allyl aldehyde mixture. Dissolve 3-(4-(tert-butyl)phenyl)acrylaldehyde in toluene and 600ml of ethylene glycol, then add 48g (3%) of p-toluenesulfonic acid to catalyze, fractionate water, and remove the bottom after the reaction 5% Na 2 CO 3 The aqueous solution was washed 1-3 times, and then back-extracted once with ether, and the organic layers were combined to obtain 1.72Kg of the mixture. Dissolve 1.72kg of the mixture with ethanol solution, add 150g of palladium carbon, put it into the reaction kettle, replace the gas in the bottle with hydrogen, pressurize to 6-7MPa and react overnight. After the reaction was complete, the palladium carbon was filtered off, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com