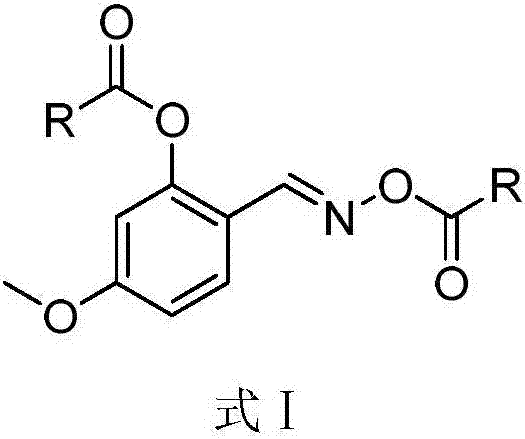
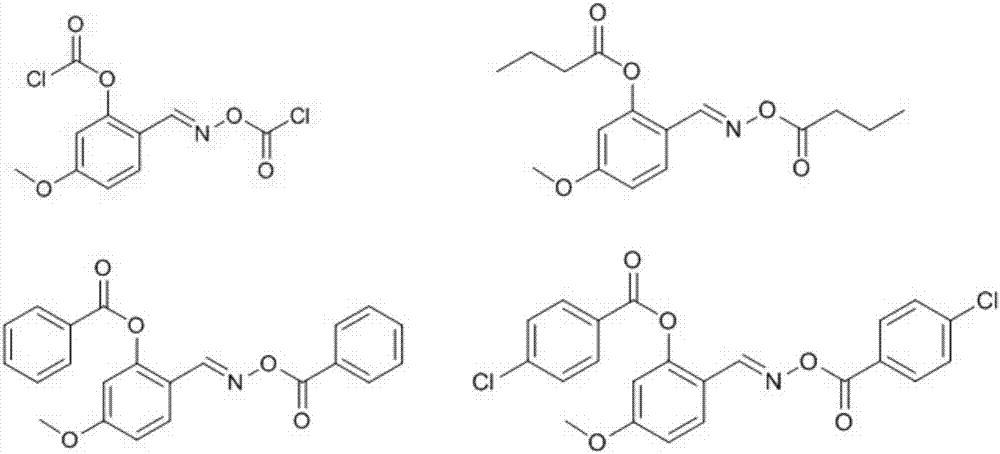
Salicylaldoxime ester compounds as well as preparation method and application thereof
A technology of ester compound and salicylaldoxime, which is applied in the field of drug synthesis, can solve the problems of lack of industrial development, insecticidal or bactericidal activity is not ideal, and achieves good market development prospects, outstanding bacteriostatic effect, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of (E)-2(((2-chloroacetoxy)imino)methyl)-5-methoxyphenyl-2-chloroacetate
[0038]
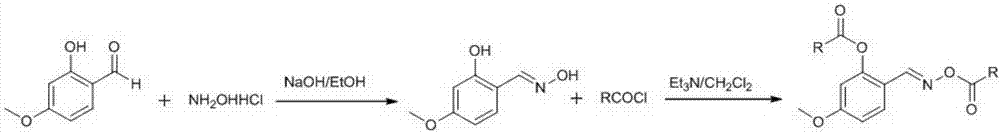
[0039] Weigh respectively 997.12mg of 4-methoxy salicylaldehyde (6.56mmol) and 548mg of hydroxylamine hydrochloride (7.89mmol) into an eggplant-shaped reaction flask, add a mixed solution of ethanol and water (22mL of ethanol, 44mL of water), Add 1.3mL of 50% NaOH at 0°C, react at room temperature for one hour after the dropwise addition, and use CH2 Cl 2 After extraction, the organic phase was washed with brine and dried over anhydrous sodium sulfate. After rotary evaporation under reduced pressure, silica gel column chromatography separated to obtain 4-methoxy salicylaldehyde hydroxylamine. White crystals, yield 71%, m.p.138.4-139.6°C. 1H NMR (DMSO-D6, 500MHz), δ: 3.73(s, 3H), 6.44~6.48(m, 2H), 7.36(d, J=8.0Hz, 1H), 8.25(s, 1H), 10.24(s , 1H), 11.07 (s, 1H); 13C NMR (DMSO-D6, 125MHz), δ: 55.65, 101.55, 106.59, 111.68, 129.92, 148.59, 158.14, 161.73. MS (ESI), m / z: 167 ...
Embodiment 2
[0042] Synthesis of (E)-2-(((butyryloxy)imino)methyl)-5-methoxyphenyl-butyrate
[0043]
[0044] Weigh 167mg of 4-methoxysalicylaldehyde hydroxylamine (1mmol) into a dry 50mL eggplant-shaped reaction bottle, add 15mL of dichloromethane, stir until the substrate is completely dissolved, add 200μL of triethylamine, and heat at 0~5℃ A solution of 127 mg of butyryl chloride (1.2 mmol dissolved in an appropriate amount of dry dichloromethane) was slowly added dropwise under ice-cooling. After the dropwise addition was completed, the mixture was followed and monitored by TLC at room temperature. When the raw material point disappeared, it was washed three times with 20 mL of saturated sodium bicarbonate, water and saturated NaCl aqueous solution, and dried over anhydrous sodium sulfate. After rotary evaporation under reduced pressure, it was separated by silica gel column chromatography to obtain (E)-2(((2-butyryloxy)imine)methyl)-5-methoxyphenyl-2-butyrate. Pale yellow oily liq...
Embodiment 3
[0046] Synthesis of (E)-2-(((benzoyloxy)imino)methyl)-5-methoxyphenyl-benzoate
[0047]
[0048] Referring to the synthetic method described in Example 1, using 4-methoxy salicylaldehyde hydroxylamine and benzoyl chloride as raw materials, prepare (E)-2(((benzoyloxy)imine)methyl)- 5-Methoxyphenyl-2-benzoate. White solid, yield 71%, m.p.136.5-137.0°C. 1 H NMR (CDCl 3 , 500MHz), δ: 8.60(s, 1H), 8.27~8.25(m, 2H), 8.14~8.11(m, 2H), 8.08~8.06(m, 2H), 7.71~7.67(m, 1H), 7.63 ~7.52(m, 2H), 7.50~7.43(m, 3H), 6.93(dd, J=9.0, 2.5Hz, 1H), 6.82(s, 1H), 3.88(s, 3H); 13 C NMR (CDCl 3 ,125MHz),δ:164.69,164.01,163.31,151.60,151.47,134.16,133.74,133.34,132.30,130.48,130.22,129.75,129.34,128.86,128.77,128.70,128.51,115.4,113.18,108.37,55.77。 ESI-MS m / z:376([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com