Preparation method of helicobacter pylori lipopolysaccharide outer-core octasaccharide
A Helicobacter pylori and octasaccharide technology, applied in the field of sugar chemistry, can solve problems such as poor repeatability of experiments, uneven molecular structure, and small amount of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
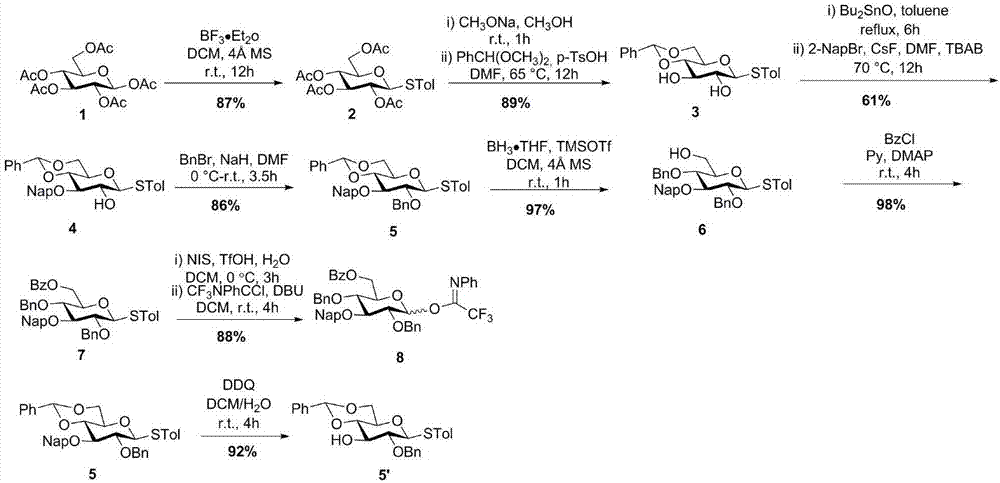
[0040] The synthesis of sugar block 8 as figure 1 :
[0041] Such as figure 1 As shown, using peracetylated glucose 1 as the starting material, boron trifluoride ether (BF 3 OEt 2 ) under the action of p-cresylthiol to generate all-acetylthioglucoside compound 2. Acetyl in sodium methoxide (NaOCH 3 ) under the action of deacetylation, exposing four hydroxyl groups. Tetrahydroxy compound and benzaldehyde dimethyl acetal (PhCH(OCH 3 ) 2 ) in p-toluenesulfonic acid (p-TsOHH 2 O) under the catalysis of generating 4,6-benzylidene protected thioglucoside 3 compounds. Selectively protect 3-OH with 2-methylnaphthalene (2-Nap) to obtain compound 4, and protect 2-OH with benzyl (Bn) to obtain compound 5. Using borane (BH 3 THF) and trimethylsilyl trifluoromethanesulfonate (TMSOTf) selectively open the 4,6-benylidene group to expose 6-OH to obtain compound 6. The 6-OH of compound 6 was protected with benzoyl (Bz) to obtain fully protected glucoglucoside compound 7. The hydrol...
Embodiment 2
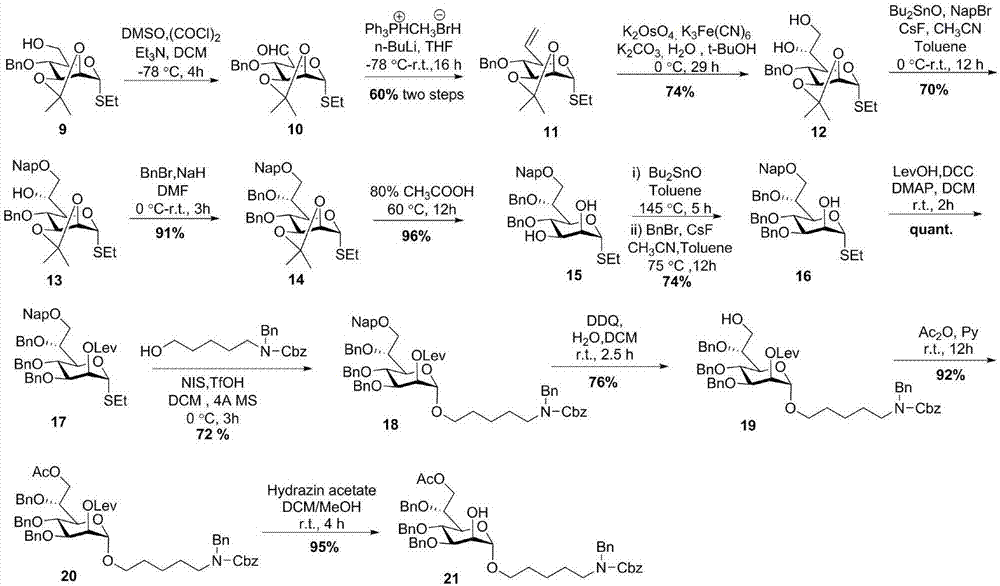
[0053] The synthesis of sugar block 21, the route is figure 2 .
[0054] Such as figure 2 As shown, using 2,3-O-propylidene-4-O-benzylmannothioside 9 as the starting material, the corresponding aldehyde compound 10 was obtained after swern oxidation. Then the carbon chain at the 6-position was extended by witting to obtain the deoxygenated olefin compound 11 at the 6-position. Olefin compounds in potassium osmate (K 2 OSo 4 ), potassium ferricyanate (K 3 Fe(CN) 6 ) and potassium carbonate (K 2 CO 3 ) under the joint action of dihydroxylation to obtain 6,7-di-hydroxyl compound 12. Using dibutyltin oxide (Bu 2 SnO) selectively protected compound 13 with Nap. 6-OH was protected with Bn to obtain compound 14. The compound 15 was obtained after removal of the propylidene group under the action of 80% acetic acid, and the 3-OH was selectively protected by Nap to obtain the heptose block 16. Compound 17 was obtained after the 2-OH of sugar block 16 was protected with Lev...
Embodiment 3
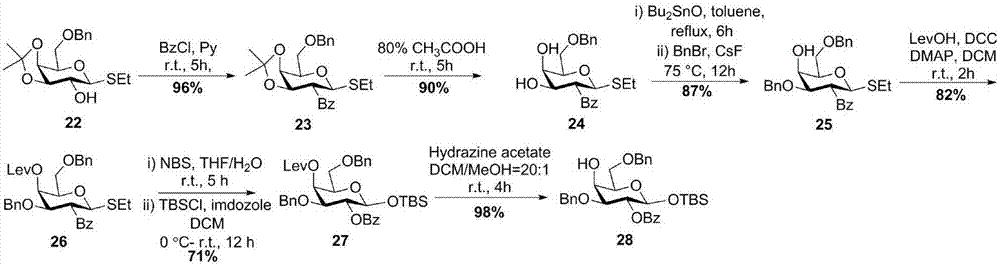
[0068] The synthesis of sugar block 28, the route is image 3 .
[0069] Such as image 3 As shown, starting from compound 22, 2-OH was protected with Bz to obtain compound 23. Compound 23 opened the propylidene group under the action of 80% acetic acid to obtain compound 24. The 3-OH of compound 24 was selectively protected with Bn to obtain compound 25. The 4-OH of compound 25 was protected with Lev to obtain compound 26. The sulfoglycoside 26 was hydrolyzed under NBS conditions, the terminal OH was exposed, and the terminal -OH was reacted with tert-butyldimethylsilyl chloride (TBSCl) to obtain galactose 27 with a fully protected terminal TBS. Lev was removed under the action of hydrazine acetate to obtain the galactose building block 28.
[0070] Specific test operation and steps:
[0071] Compound 23: Compound 22 (4.2 g, 11.8 mmol) was dissolved in pyridine (59.5 mL), and stirred at 0° C. for 5 min. BzCl (2.78 mL, 24 mmol) and a catalytic amount of DMAP were added....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com