Method and equipment thereof for synthesizing methyl isobuthl ketone with acetone two-step method
A technology of methyl isobutyl ketone and acetone, which is applied in the field of synthesis of methyl isobutyl ketone, can solve the problems of reduced operating pressure of catalytic rectification tower, increased process complexity, increased by-product generation, etc., and achieves an improved balance Improve the conversion rate, reduce energy consumption, and solve the effect of low oil-water separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
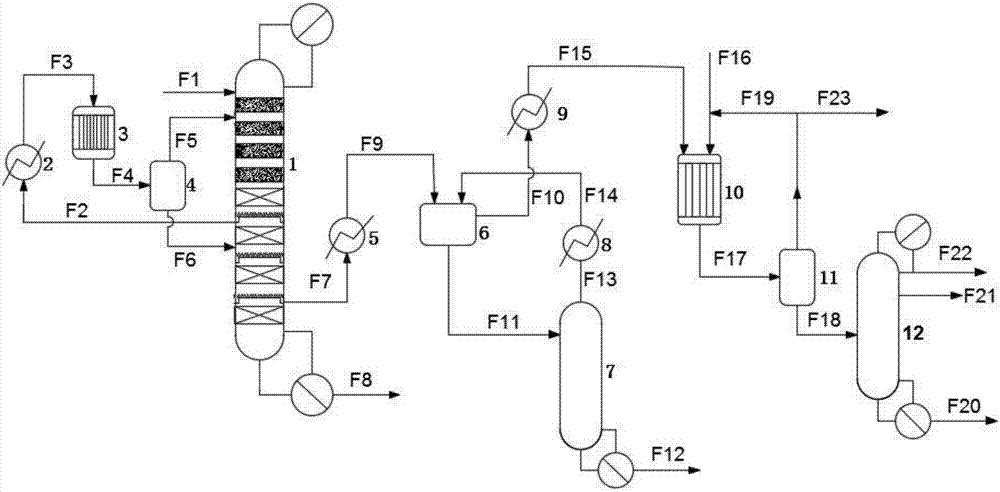
[0056] use as figure 1 The technological process of the synthetic methyl isobutyl ketone of shown acetone two-step method.
[0057] The upper part of the catalytic rectification tower is provided with four stages of heterogeneous catalyst layers, and the lower part is provided with six stages of random packing layers.
[0058] The specification of the random packing is θ ring (Ф3), the number of theoretical plates of the packing is 42, and the heterogeneous catalyst is the acidic cation exchange resin Amberlyst 15.
[0059] The acetone raw material F1 enters the catalytic rectification tower from the top of the tower, and the DAA-containing material F2 is extracted from the side line between the third and fourth stages of the catalytic rectification tower. The liquid phase output F6 of the acetone flasher is circulated back to the tower between the fourth and fifth sections of the catalytic rectification tower, and the stream F7 rich in MSO and water is sent to the fifth and ...
Embodiment 2
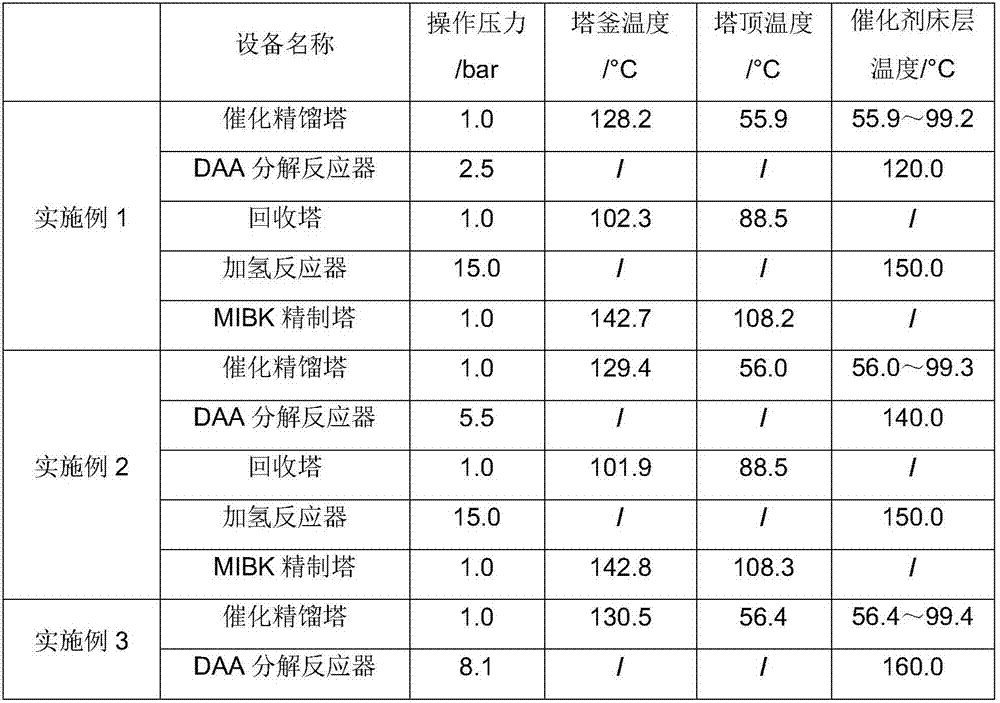
[0075] The difference from Example 1 is that the DAA decomposition reaction temperature in Example 2 is 140° C. and the pressure is 5.5 bar, and the others are the same as those described in Example 1.
[0076] The content of DAA in F7 produced from the side stream product of the catalytic rectification tower: 5.6%;
[0077] Water content in the organic phase discharge F10 of the phase separator: 5.9%;
[0078] MSO content in the aqueous phase discharge F11 of the phase separator: 4.7%;
[0079] The MIBK product F21 is extracted through the side stream, and its purity is 99.55% through gas chromatography analysis.
[0080] See Table 1 and Table 2 for other results.
Embodiment 3
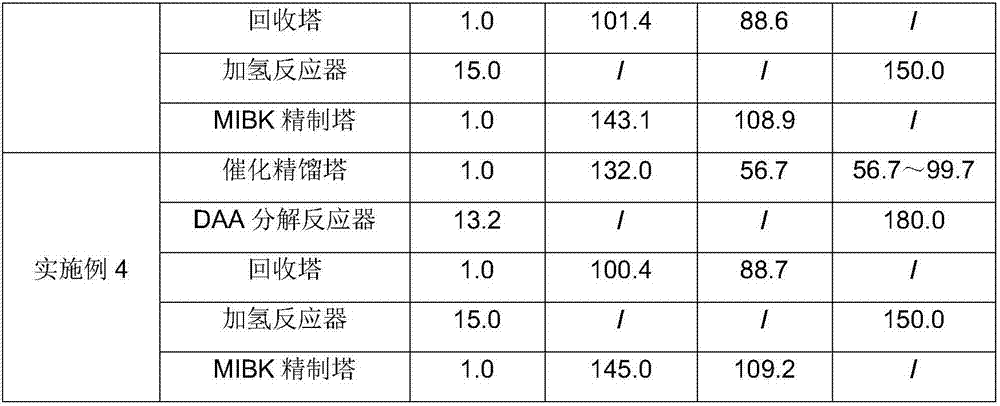
[0082] The difference from Example 1 is that the DAA decomposition reaction temperature in Example 3 is 160° C., the pressure is 8.1 bar, and the others are the same as those described in Example 1.
[0083] The content of DAA in F7 produced from the side stream product of the catalytic rectification tower: 2.2%;
[0084] Water content in the organic phase discharge F10 of the phase separator: 1.8%;
[0085] MSO content in the aqueous phase discharge F11 of the phase separator: 1.5%;
[0086] The MIBK product F21 is extracted through the side stream, and its purity is 99.72% through gas chromatography analysis.
[0087] See Table 1 and Table 2 for other results.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com