Preparation gs115/ac-amp2 for inhibiting postharvest blue mold in pear fruit
A GS115, ac-amp2 technology, applied in the direction of virus/phage, microorganism-based method, application, etc., can solve the problem that the biological control effect of antagonistic microorganisms cannot be reached or approached, and achieves inhibition of penicillium. Occurrence and prolong shelf life , the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, recombinant expression vector construction
[0041] 1. Experimental materials:
[0042] Restriction enzymes: Xho I and Not I, and T4 ligase;
[0043] Axygen DNA Gel Recovery Kit;
[0044] 2×Phanta Max Master Mix;
[0045] Escherichia coli competent cell DH5α;
[0046] Plasmid pPICZαA.
[0047] 2. Processing:
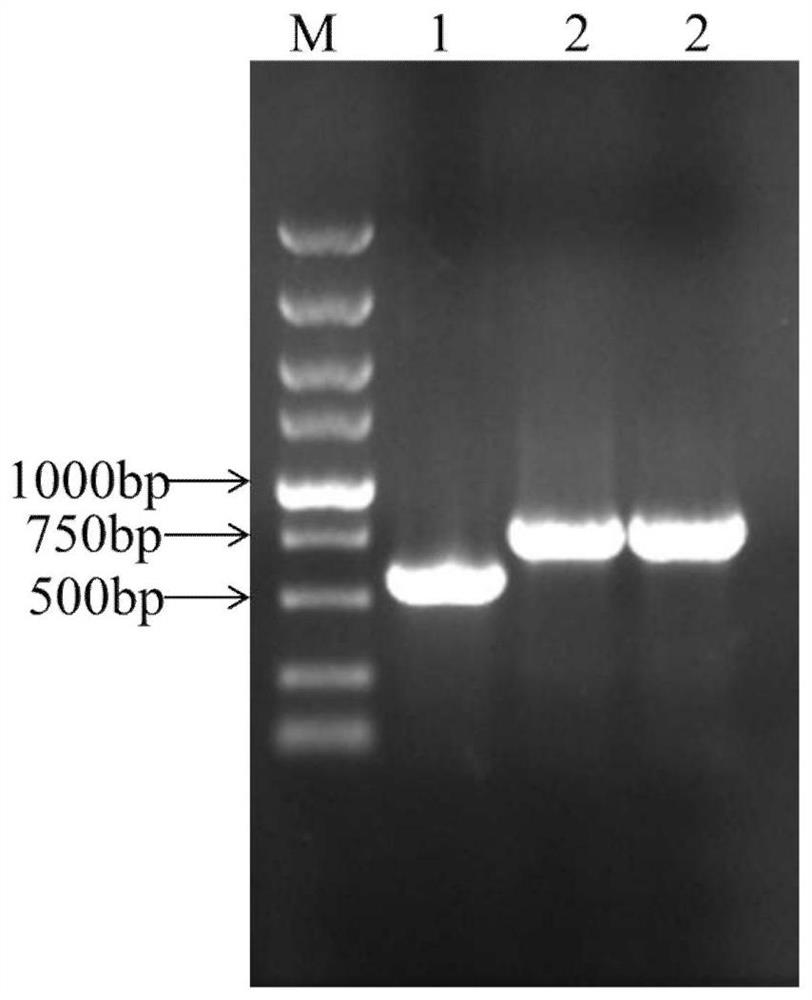
[0048] (1) After double digestion of the plasmid pPICZα A and the target gene (optimized Ac-AMP2 gene, described in SEQ ID NO: 1) containing Xho I and Not I restriction sites, the double digestion product was washed with 1% The optimized target fragment and expression vector were detected by agarose gel electrophoresis and recovered with a DNA gel recovery kit. The recovered target fragment was ligated by T4 ligase at 4°C for 16 hours.
[0049] The double enzyme digestion system is:
[0050]
[0051] After recovering the target fragment and the linearized expression vector, the reaction system for overnight ligation is:
[0052]
[0053...
Embodiment 2
[0064] Example 2. Transformation and Identification of Recombinant GS115 / Ac-AMP2 Yeast
[0065] 1. Experimental materials:
[0066] Recombinant expression vector pPICZα A / Ac-AMP2
[0067] Plasmid pPICZαA
[0068] Pichia pastoris GS115
[0069] Restriction enzyme Sac I
[0070] 2. Processing:
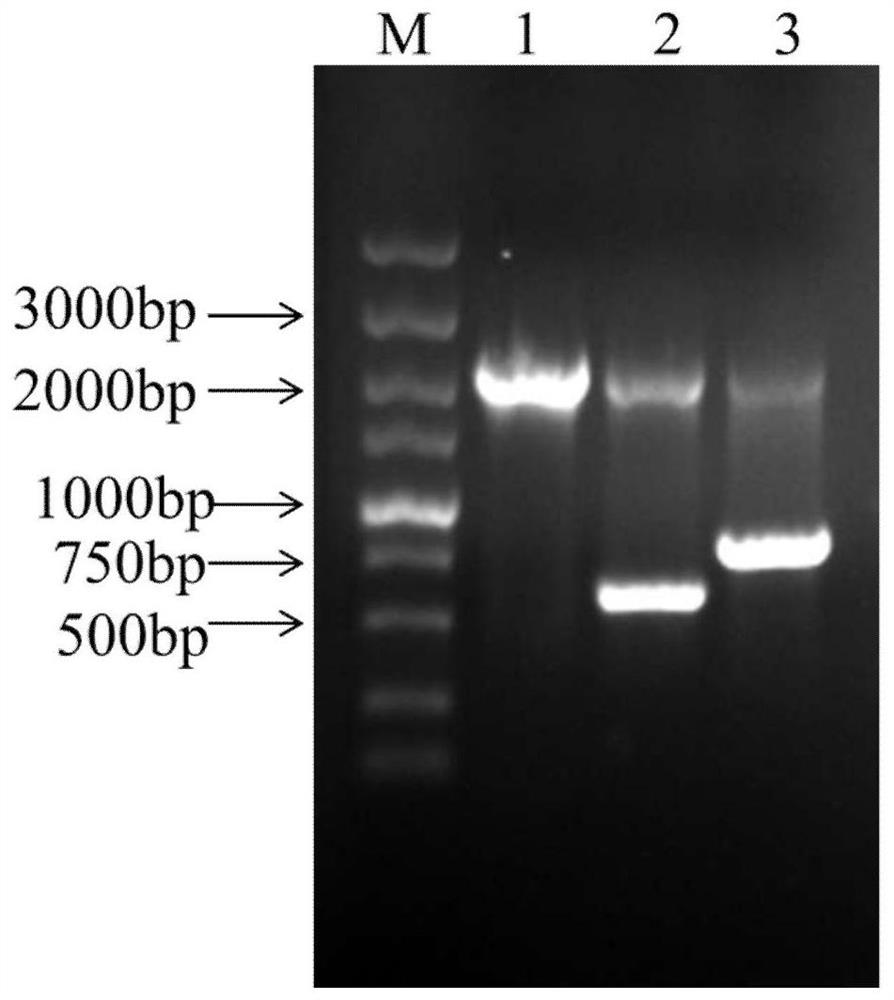
[0071] (1) The recombinant expression vector pPICZα A / Ac-AMP2, which was verified and successfully constructed above, and the empty vector pPICZα A without antimicrobial peptides were digested with Sac I.
[0072] (2) Mix 10 μL of the linearized carrier with 80 μL of the prepared GS115 competent cells and transfer them to the electroporation cup, transform them into competent yeast cells by electric shock according to the program set by the Bio-Rad electroporation instrument, and quickly add 1 mL of 1M Sorbitol, let stand at 28°C for 1 h, take 200 μl of electroporation transformation solution and spread evenly on YPD+Zeocin resistance plate (Zeocin concentration 100 μg / mL), and cult...
Embodiment 3
[0079] Example 3, Expression and Identification of Ac-AMP2 in Yeast
[0080] 1. Expression level of Ac-AMP2 in recombinant yeast GS115 / Ac-AMP2
[0081] 1. Experimental materials:
[0082] Recombinant Yeast GS115 / Ac-AMP2
[0083] Bradford protein concentration assay kit;
[0084] 2. Processing:
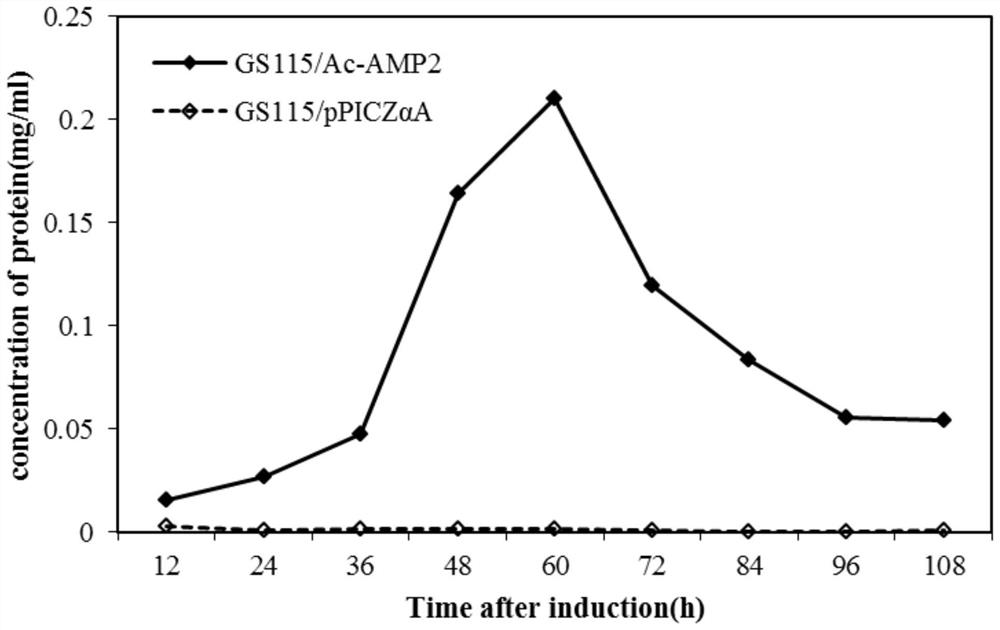
[0085] (1) Pick a single colony of the recombinant strain GS115 / Ac-AMP2 and the empty plasmid control strain GS115 / pPICZα A that has been verified that the target gene has been integrated into the yeast genome, and induce expression. Methanol was added every 24 hours to a final concentration of 1% and continued to induce for 108 hours; every 12 hours, 1 mL samples were taken and preserved to determine the expression level of antimicrobial peptides.
[0086] (2) The sample was centrifuged at 8000 g for 10 min, and the supernatant and precipitate were collected respectively. At each sampling time point, 50 uL of the supernatant was taken to detect the protein concentration in the sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com