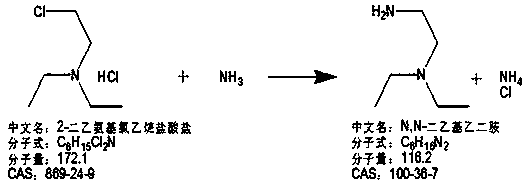
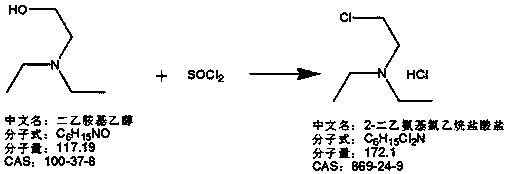
Preparation method of N,N-diethylenediamine
A technology of diethylethylenediamine and diethylaminoethanol, which is applied in the field of preparation of N,N-diethylethylenediamine, can solve the problems of unfavorable industrial production, high cost of raw materials, cumbersome operation, etc., and achieve favorable The effect of industrialization promotion, reduction of production cost, and high operational safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] (1) Preparation of 2-diethylaminoethyl chloride hydrochloride:
[0064] 146g thionyl chloride and 240g dichloromethane are added into the 1L reaction flask to lower the temperature, and a mixed solution of 120g of diethylaminoethanol and 100g of dichloromethane is prepared in a 500ml beaker during the cooling process. When the temperature in the reaction flask drops to - At 10°C, start to add the mixture of diethylaminoethanol and dichloromethane dropwise while maintaining the temperature at -10~20°C, and complete the dropwise addition in 1 hour and 35 minutes;
[0065] After the dropwise addition was completed, the temperature was raised to 20-45°C for 3 hours of heat preservation and reaction. After the heat preservation was completed, the methylene chloride was concentrated and concentrated for 55 minutes to the point where no liquid emerged. A total of 265 g of dichloromethane was recovered. The recovered dichloromethane can be used directly;
[0066] After concentr...
Embodiment 2
[0076] (1) Preparation of 2-diethylaminoethyl chloride hydrochloride:
[0077] 146g thionyl chloride and 240g dichloromethane are added into the 1L reaction flask to lower the temperature, and a mixed solution of 120g of diethylaminoethanol and 100g of dichloromethane is prepared in a 500ml beaker during the cooling process. When the temperature in the reaction flask drops to - 10°C, start to add the mixture of diethylaminoethanol and dichloromethane dropwise while maintaining the temperature at -10~20°C, and complete the dropwise addition in 1 hour and 28 minutes;
[0078] After the dropwise addition was completed, the temperature was raised to 20-45°C for 5 hours of heat preservation and reaction. After the heat preservation was completed, the dichloromethane was concentrated until it was concentrated for 1 hour and 05 minutes. A total of 258g of dichloromethane was recovered, and the recovered dichloromethane could be used directly;
[0079] After concentrating to dryness, ...
Embodiment 3
[0089] (1) Preparation of 2-diethylaminoethyl chloride hydrochloride:
[0090] 260g thionyl chloride and 240g reclaim dichloromethane and join in 1L reaction flask and cool down, prepare the mixed solution of diethylaminoethanol 120g and dichloromethane 100g with 500ml beaker in the cooling process, when the temperature in the reaction flask drops to -10°C, start to maintain the temperature at -10~20°C and add the mixture of diethylaminoethanol and dichloromethane dropwise, and the dropwise addition is completed in 1 hour and 32 minutes;
[0091] After the dropwise addition was completed, the temperature was raised to 20-45°C for 2 hours of heat preservation and reaction. After the heat preservation was completed, dichloromethane was concentrated and concentrated for 58 minutes to the point where there was no water left. A total of 245 g of dichloromethane was recovered, and the recovered dichloromethane could be used directly;
[0092] After concentrating to dryness, cool dow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com