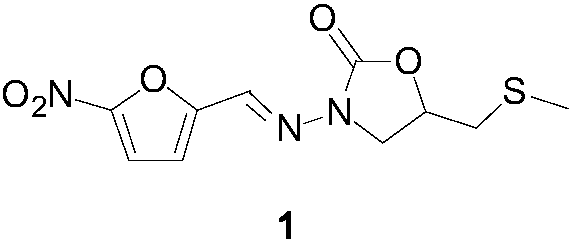
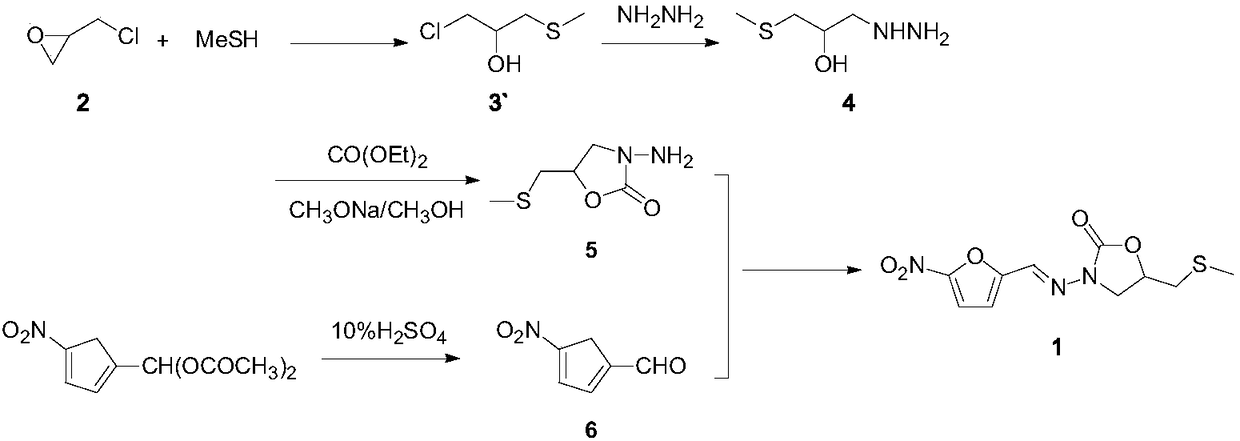
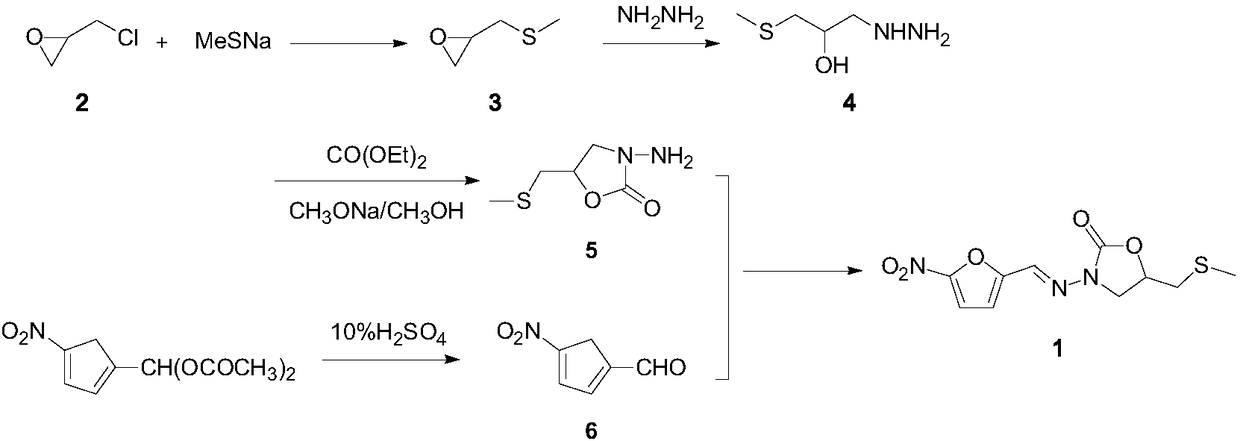
Nifuratel preparation method
A technology of nifuratel and molar ratio, applied in the field of preparation of nifuratel, can solve the problems of high risk of sodium hydride, many side reactions, unstable aldehyde groups, etc., achieves cheap and easy availability of raw materials and reagents, and improved purity and yield, the effect of improving the production environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of Compound 4`:
[0058] In a 1000ml reaction flask, add 264.00g of tert-butyl carbazate and 260ml of purified water, raise the temperature of the system to 60°C, add 104.05g of glycidyl methyl sulfide dropwise at this temperature, control the rate of addition, and make the reaction system The temperature does not exceed 65°C. After the dropwise addition, raise the temperature to 80°C and keep the temperature for 4 hours. After the heat preservation is completed, cool to room temperature and continue to cool down to 5°C. Solids are precipitated, filtered with suction, rinsed with cold water, and dried to obtain 212.30g Off-white solid, that is, compound 4', with a yield of 89.80% and a purity of 98.5%.
Embodiment 2
[0060] Preparation of Compound 4`:
[0061] In a 1000ml reaction flask, add 159.00g of tert-butyl carbazate and 260ml of purified water, raise the temperature of the system to 60°C, add 104.02g of glycidyl methyl sulfide dropwise at this temperature, control the rate of addition, and make the reaction system The temperature does not exceed 65°C. After the dropwise addition, raise the temperature to 80°C and keep the temperature for 8 hours. After the heat preservation is completed, cool to room temperature and continue to cool down to 5°C. Solids are precipitated, filtered with suction, rinsed with cold water, and dried to obtain 203.12g Off-white solid, that is, compound 4', with a yield of 86.00% and a purity of 98.7%.
Embodiment 3
[0063] Preparation of Compound 5`:
[0064] In a 2000ml reaction flask, add 200.00g of compound 4 of Example 1, add DMF 300ml, cuprous bromide 7.32g, urea 76.27g, heat up to 70°C, keep warm for 8h, after the heat preservation is completed, cool to room temperature, stir Add 600ml of water dropwise, continue to cool down to below 10°C after the dropwise addition, and filter with suction to obtain 195.30g of solid, namely compound 5', with a yield of 87.83% and a purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com