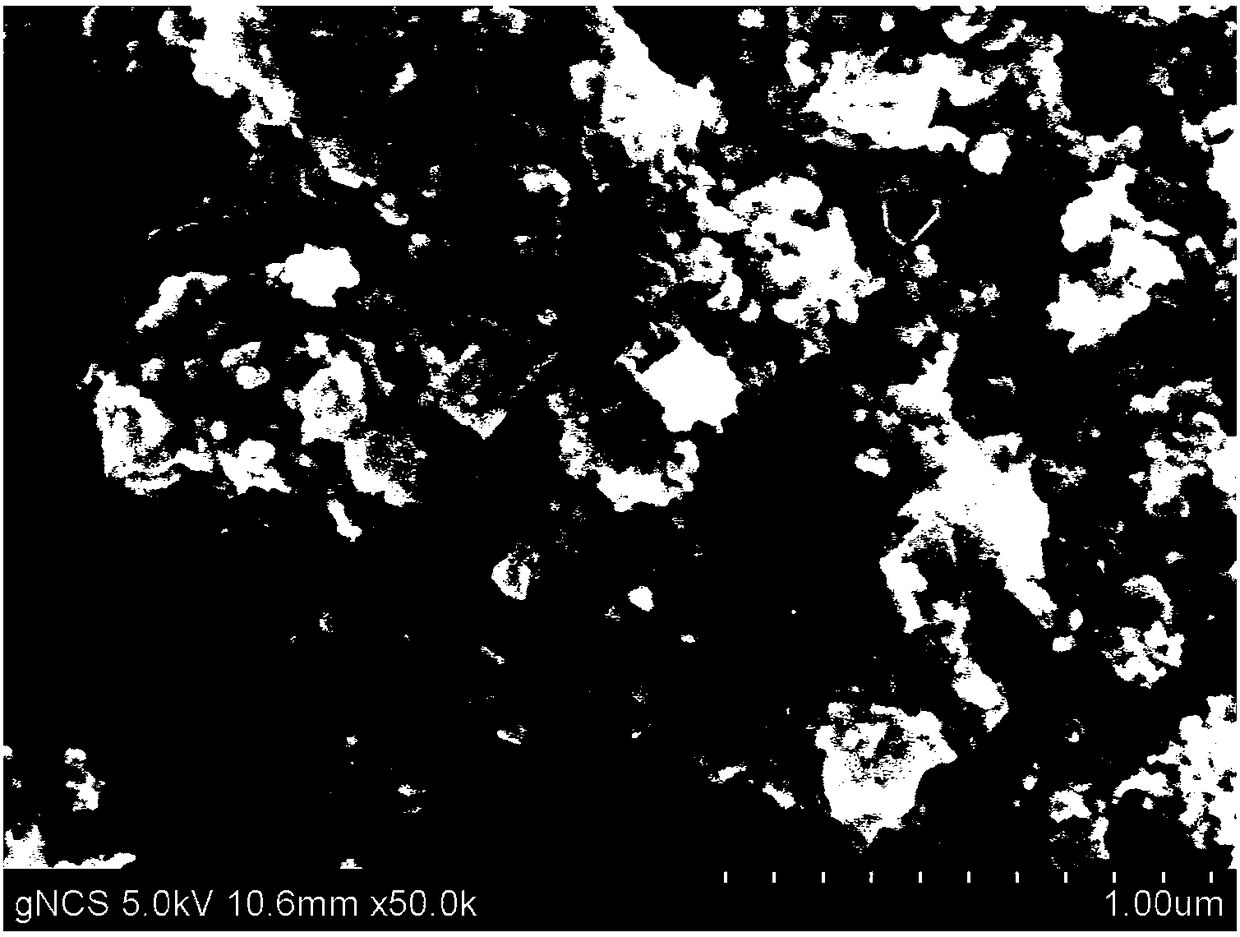
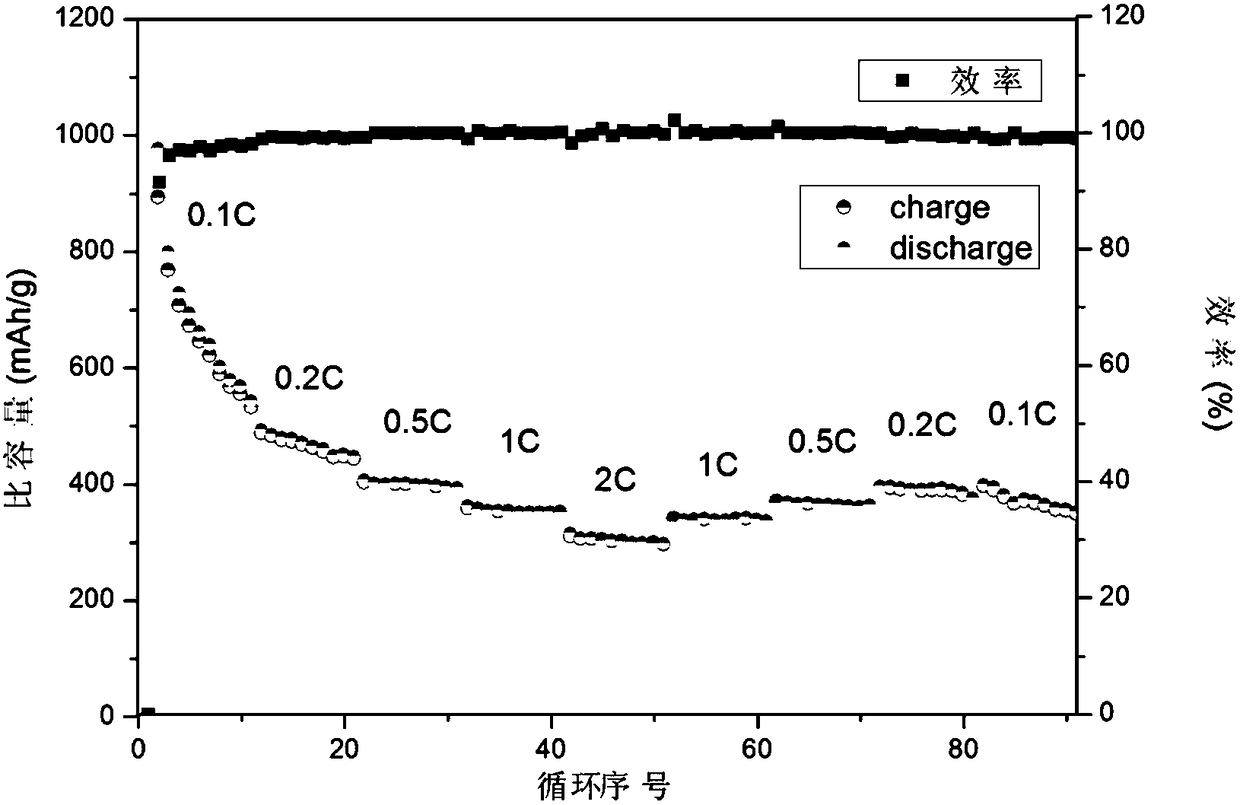
Preparing method of nanometer nickel-cobalt-sulphur particles serving as positive electrode of lithium sulphur battery
A lithium-sulfur battery and nano-nickel technology, which is applied in the field of preparation of nano-nickel-cobalt-sulfur particles, can solve the problems of high battery sealing performance, damage to the battery structure, positive electrode volume expansion, etc., to reduce the shuttle effect, increase the specific surface area, small size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Weigh 75 mg of cobalt nitrate hexahydrate, 75 mg of nickel nitrate hexahydrate and 3 g of sodium acetate and dissolve in 40 mL of deionized water, then add 0.2 g of potassium persulfate or ammonium persulfate and stir for 30 min to obtain a pink solution.
[0031] (2) Pour the pink solution obtained in step (1) into the lining of a 50mL polytetrafluoroethylene autoclave, seal it, place it in a constant temperature oven, and raise the temperature to 180°C at a rate of 10°C / min for 6 hours. , after being naturally cooled, washed with deionized water and ethanol respectively, centrifuged several times until there were no foreign ions, and placed in a constant temperature vacuum oven at 60°C for 12 hours to obtain a fluffy and dry black nickel cobaltate nanopowder.
[0032] (3) Take 30 mg of black nickel cobaltate nanopowder in step (2), add 40 mL of deionized water and ultrasonically disperse for 30 minutes, add 0.96 g of sodium sulfide nonahydrate, and pour the mixture...
Embodiment 2
[0035] (1) Weigh 100 mg of cobalt nitrate hexahydrate, 50 mg of nickel nitrate hexahydrate and 3 g of sodium acetate and dissolve in 40 mL of deionized water, then add 0.2 g of potassium persulfate or ammonium persulfate and stir for 60 min to obtain a pink solution.
[0036] (2) Pour the pink solution obtained in step (1) into the lining of a 50mL polytetrafluoroethylene autoclave, seal it, place it in a constant temperature oven, and raise the temperature to 180°C at a rate of 10°C / min for 6 hours. , after natural cooling, wash with deionized water and ethanol respectively, centrifuge several times until there are no foreign ions, and place in a constant temperature vacuum oven at 60°C for 12 hours to obtain fluffy and dry black nickel cobaltate nanopowder.
[0037] (3) Take 30 mg of black nickel cobaltate nanopowder in step (2), add 40 mL of deionized water and ultrasonically disperse for 30 minutes, add 0.96 g of sodium sulfide nonahydrate, and pour the mixture into the lin...
Embodiment 3
[0039] (1) Weigh 50 mg of cobalt nitrate hexahydrate, 100 mg of nickel nitrate hexahydrate and 3 g of sodium acetate and dissolve in 40 mL of deionized water, then add 0.2 g of potassium persulfate or ammonium persulfate and stir for 60 min to obtain a pink solution.
[0040] (2) Pour the pink solution obtained in step (1) into the lining of a 50mL polytetrafluoroethylene autoclave, seal it, place it in a constant temperature oven, and raise the temperature to 180°C at a rate of 10°C / min for 6 hours. , after natural cooling, wash with deionized water and ethanol respectively, centrifuge several times until there are no foreign ions, and place in a constant temperature vacuum oven at 60°C for 12 hours to obtain fluffy and dry black nickel cobaltate nanopowder.
[0041](3) Take 30 mg of black nickel cobaltate nanopowder in step (2), add 40 mL of deionized water and ultrasonically disperse for 30 minutes, add 0.96 g of sodium sulfide nonahydrate, and pour the mixture into the lini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Coulombic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com