LC-MS (Liquid Chromatograph-Mass Spectrometer) quantitative method for detecting protein drug in body on basis of label small molecule exclusive separation-amplification and application of protein drug
A protein drug and small molecule technology, which is applied in the field of medicine, can solve the problems that the detection sensitivity cannot meet the detection of low-concentration protein drugs, the proteolysis takes a long time, and the operation requirements are high, so as to overcome the bottleneck of protein drug concentration, improve the detection sensitivity, and repeat the process. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of functionalized gold nanoprobes:
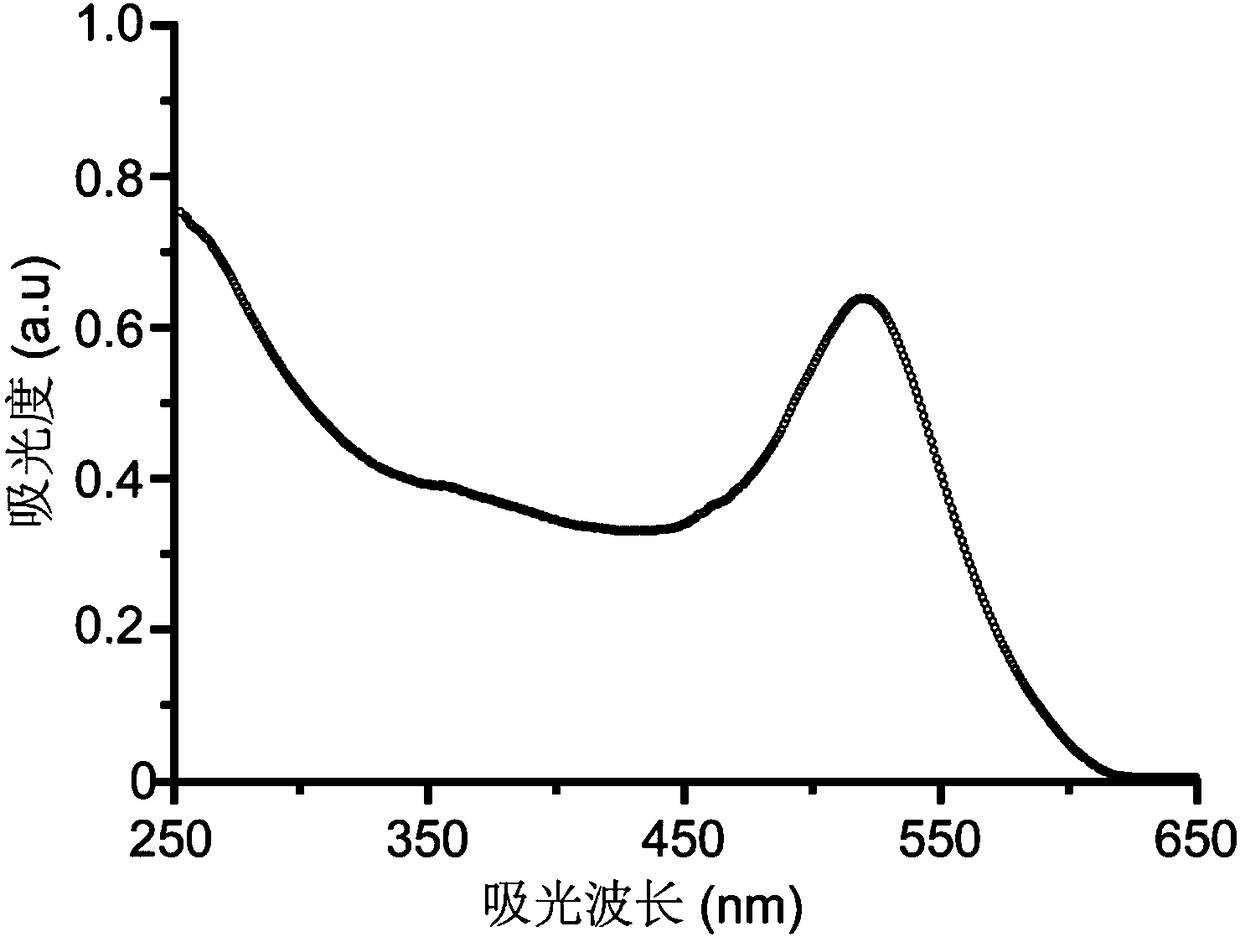
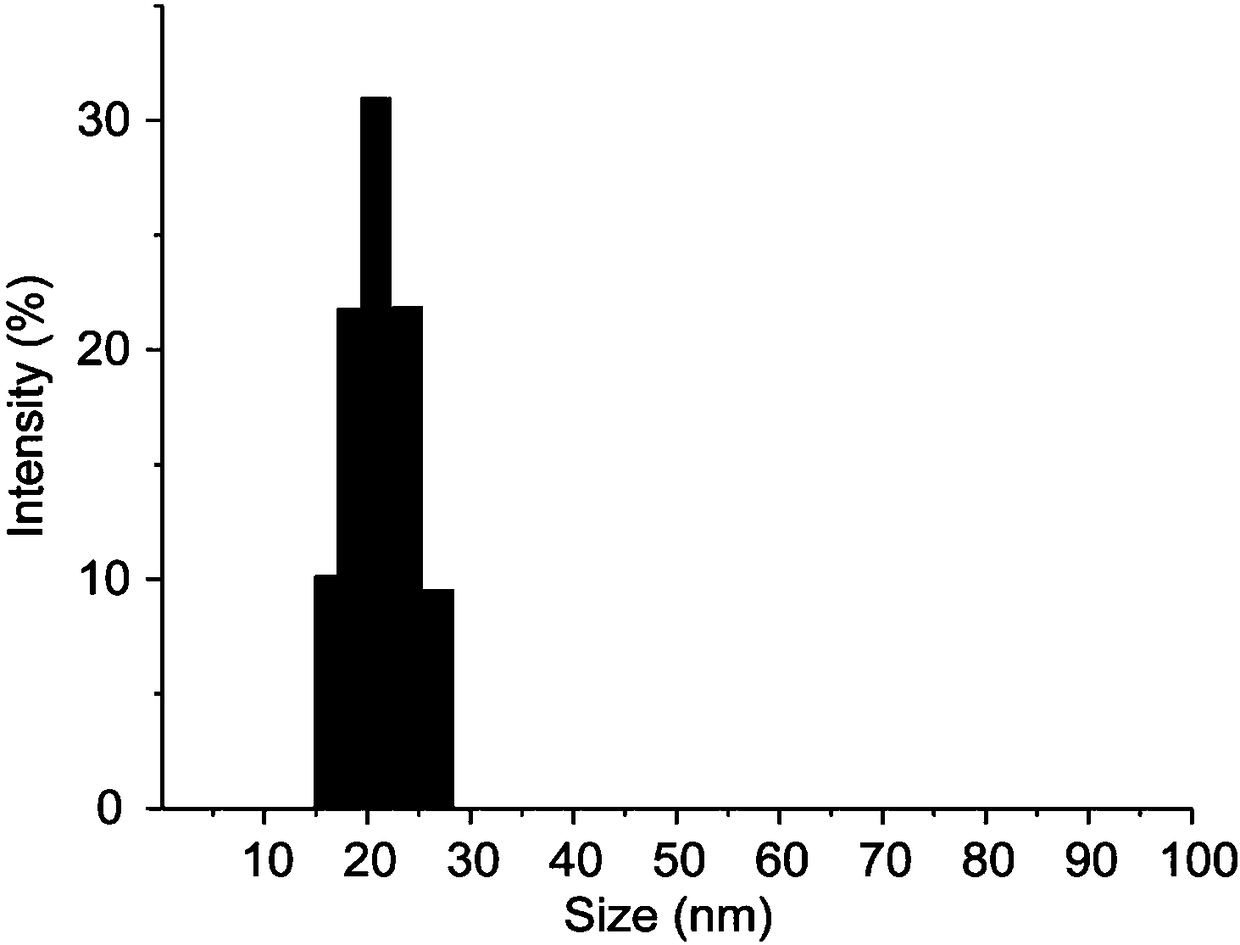
[0043]Weigh an appropriate amount of chloroauric acid and dissolve it with ultrapure water to prepare 100mL of 1mM chloroauric acid aqueous solution, transfer it to a round bottom flask and heat to boiling, then quickly add 10mL of the prepared 38.8mM sodium citrate aqueous solution, and continue to boil and reflux for 15 minutes, remove the heat source, continue to reflux, stir and cool to room temperature to obtain a gold nanoparticle solution. Take out 1 mL of the gold nanoparticle solution, add 60 μL of 50 μg / mL adenosine aqueous solution, shake and mix at room temperature for 30 minutes to obtain the gold nanoparticle / adenosine complex. Activate the aptamer with TCEP 50 times that of the thiol aptamer at room temperature for 1 hour, pipette the nano-gold / adenosine complex into the activated thiol aptamer solution, shake it at room temperature in the dark for 16 hours, and add it dropwise 10 μL of Tris-acetic acid bu...
Embodiment 2
[0045] Preparation of functionalized magnetic bead probes:
[0046] Weigh silicon amide-coated magnetic beads, ultrasonically disperse and wash them several times with ultrapure water, and finally disperse in 10 mM PBS buffer solution (pH, 8.0), add glutaraldehyde aqueous solution to the system, and shake at room temperature for 1.5 hours. Apply an external magnetic field to remove the supernatant, add 3×SSC buffer to redisperse, add excess aminated aptamer solution, shake well at room temperature and react overnight. Add sodium borohydride aqueous solution to react for 0.5 hours, wash with 3×SSC buffer solution, 0.2% SDS aqueous solution, and finally wash with ultrapure water for 3 times, remove the supernatant with an external magnetic field, and disperse in 10mM Tris-HCl buffer solution (pH, 7.6).
Embodiment 3
[0048] The formation of the double-wrapped sandwich structure:
[0049] The functionalized magnetic bead probe was repeatedly washed 3 times with PBS buffer (10mM, pH, 7.4), and the supernatant was removed by an external magnetic field, and then redispersed in a K-containing solution. + , Mg 2+ In the activation buffer system, after activation at room temperature for 1 hour, add the sample to be tested containing thrombin, place it at 37°C for 2 hours, mix and react at a constant temperature, remove the supernatant, and prepare the sample containing K + , Mg 2+ and 0.005% Tween-20 (w / v) washing buffer for gentle washing 3 times, and the supernatant was removed. After the functionalized gold nanoprobe was centrifuged to remove the supernatant, it was redispersed in a solution containing K + , Mg 2+ In the activation buffer system, and transferred to the magnetic bead probe sample after removing the supernatant, placed at 37°C for a constant temperature mixing reaction for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com