Preparation method and application of fused bovine antibacterial peptide and interleukin 2 co-expression recombinant yeast preparation
A technology of interleukins and antimicrobial peptides, applied in the biological field, can solve problems such as the emergence of drug resistance of bacteria, hindering the development of aquaculture, and the failure of drugs to treat antibiotics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
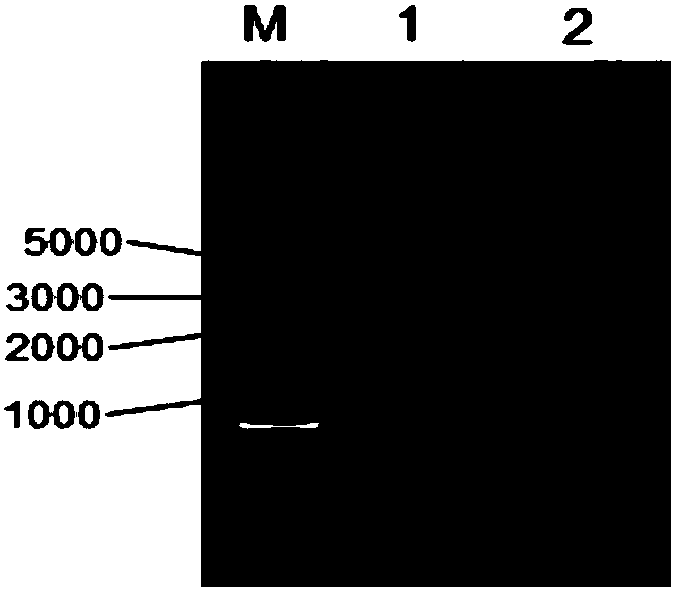
[0120] Embodiment 1, bovine antimicrobial peptide interleukin 2 fusion protein (FBAPIL2) and its coding gene
[0121] 1. Acquisition of the fusion protein FBAPIL2 and its coding gene
[0122] The bovine antimicrobial peptide and interleukin 2 were fused to obtain a fusion protein named FBAPIL2.
[0123] The amino acid sequence of the fusion protein is shown as sequence 1 in the sequence listing, the fusion gene encoding the fusion protein FBAPIL2 is named FBAPIL2, and the nucleotide sequence of the fusion gene is sequence 2.
[0124] Among them, the 265th-353rd position of the sequence 1 is a bovine antibacterial peptide, the 155th-264th position is a connecting peptide, and the 1st-154th position is an interleukin 2;
[0125] The 793-1059th position of sequence 2 is the bovine antimicrobial peptide coding nucleic acid, the 463-792nd position is the connecting peptide coding nucleic acid, and the 1-462nd position is the interleukin-2 coding nucleic acid.
[0126] The above-m...
Embodiment 2
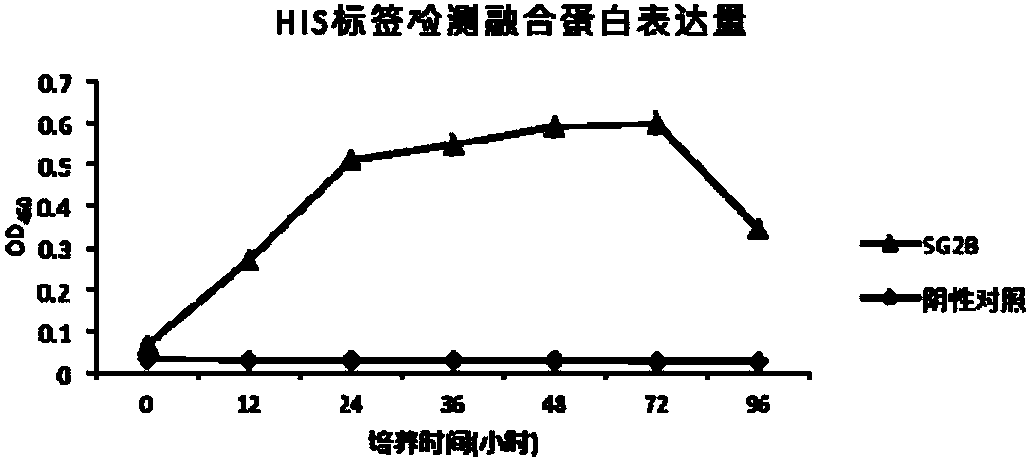
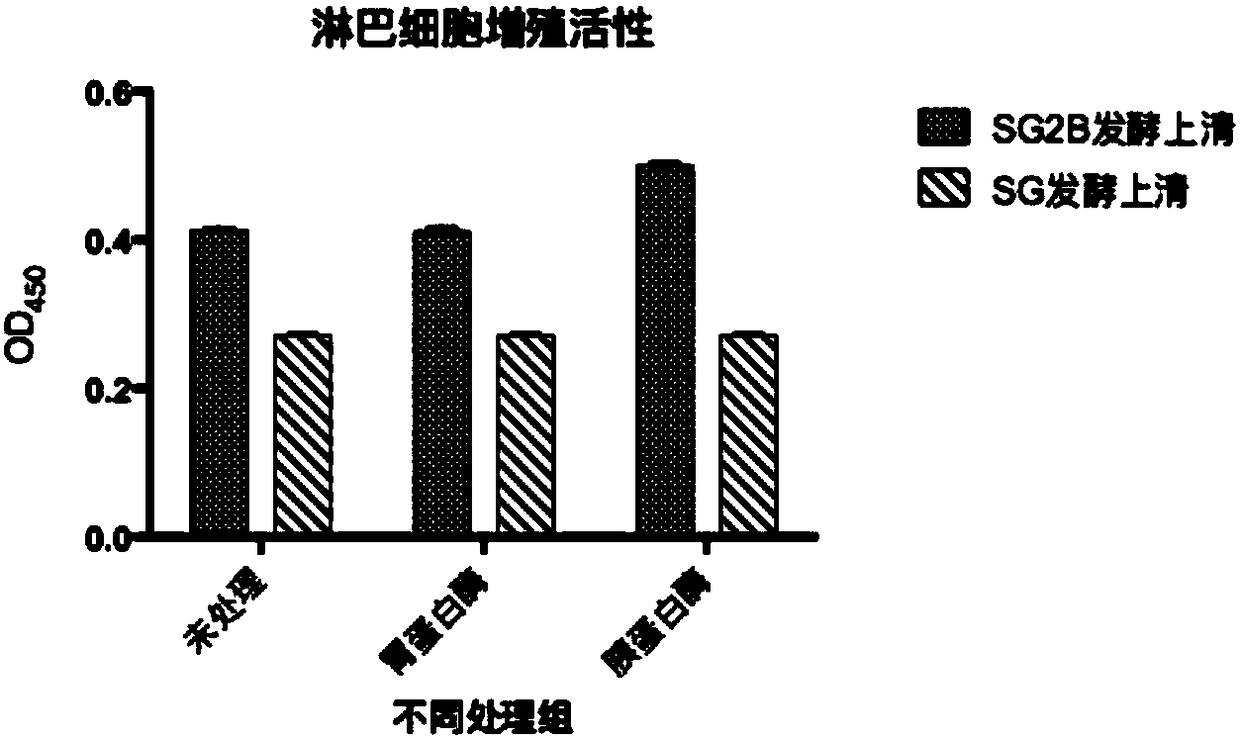
[0146] Embodiment 2, the influence of bovine antimicrobial peptide interleukin 2 fusion protein (FBAPIL2) on lymphocyte proliferation
[0147] 1. Preparation of recombinant bacteria SMDpG-2B fermentation supernatant
[0148] (1) the recombinant bacterium SMDpG-2B (hereinafter referred to as SG2B) that embodiment 1 obtains is inoculated in 3mL substratum 1 (substratum 1 is the liquid medium that adds bleomycin (Zeocin) to obtain in YPD substratum, and The concentration of bleomycin was 100mg / mL), and the activated strain was cultivated overnight at 28°C and 200rpm.
[0149] (2) Take 300 μL of the bacterial solution obtained in step (1) and inoculate it into a 100 mL Erlenmeyer flask containing 30 mL of YPD medium, ferment at 28 °C and 200 rpm on a shaker for 48 hours (OD 600 for 25).
[0150] (3) Take 5 mL of the bacterial liquid obtained in step (2), centrifuge at 12 000×g for 2 min, and name the obtained supernatant as SG2B fermentation supernatant.
[0151] 2. Protease tr...
Embodiment 3
[0168] Example 3, Bacteriostatic Activity Detection of Bovine Antibacterial Peptide Interleukin 2 Fusion Protein (FBAPIL2)
[0169] Determination of bovine antimicrobial peptide interleukin 2 fusion protein FBAPIL2 on Escherichia coli standard bacteria (G - ) (hereinafter referred to as S-G - ), Escherichia coli resistant bacteria (G - ) (hereinafter referred to as R-G - ), Staphylococcus aureus standard bacteria (G + ) (hereinafter referred to as S-G + ), drug-resistant Staphylococcus aureus (G + ) (hereinafter referred to as R-G + ) antibacterial situation, the specific method is as follows:
[0170] First, the four bacterial strains were inoculated and activated and cultured in the exponential growth phase (OD 600 about 0.5), then diluted to OD with LB medium 600 About 0.005, the diluted bacterial solution was inoculated on a 96-well cell culture plate, 100 μL / well, one kind of bacteria per 96-well cell culture plate.
[0171] For each 96-well cell culture plate co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com