Anti-tumor prodrug with P-glycoprotein inhibition function and preparation method
An anti-tumor drug, glycoprotein technology, applied in the direction of anti-tumor drugs, pharmaceutical formulations, medical preparations with non-active ingredients, etc., can solve the problems of slow release of active drugs, reduced efficacy of chemotherapy drugs, etc. The effect of long circulation, increasing drug solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
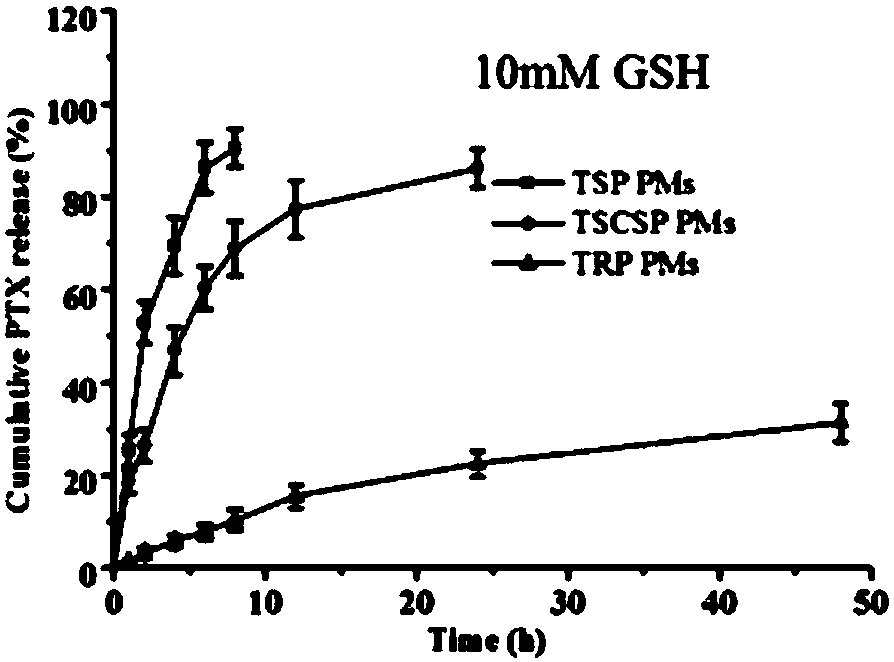
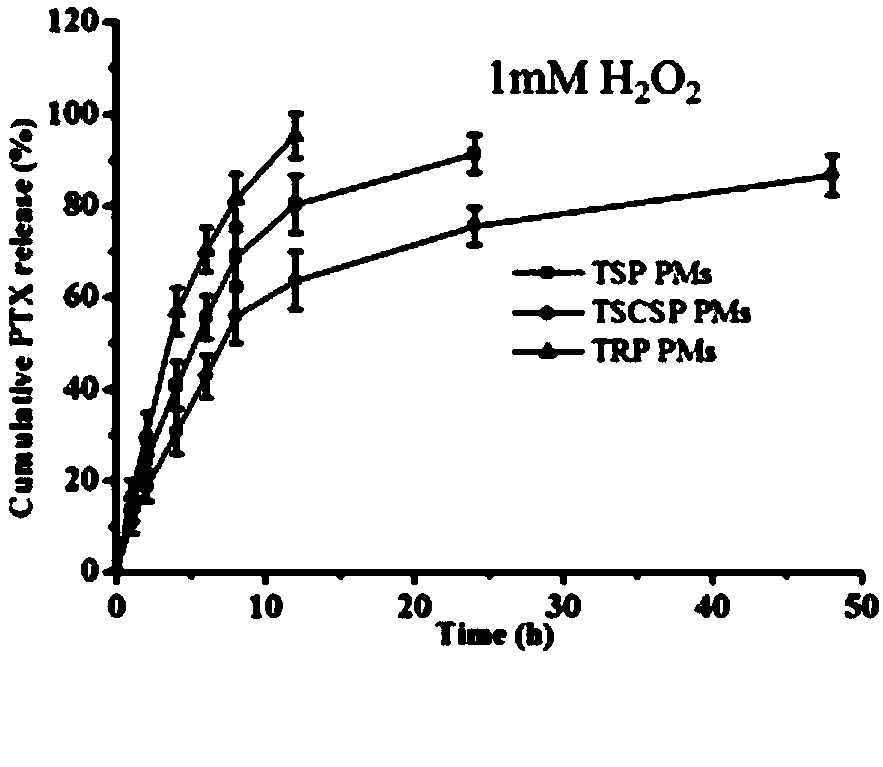
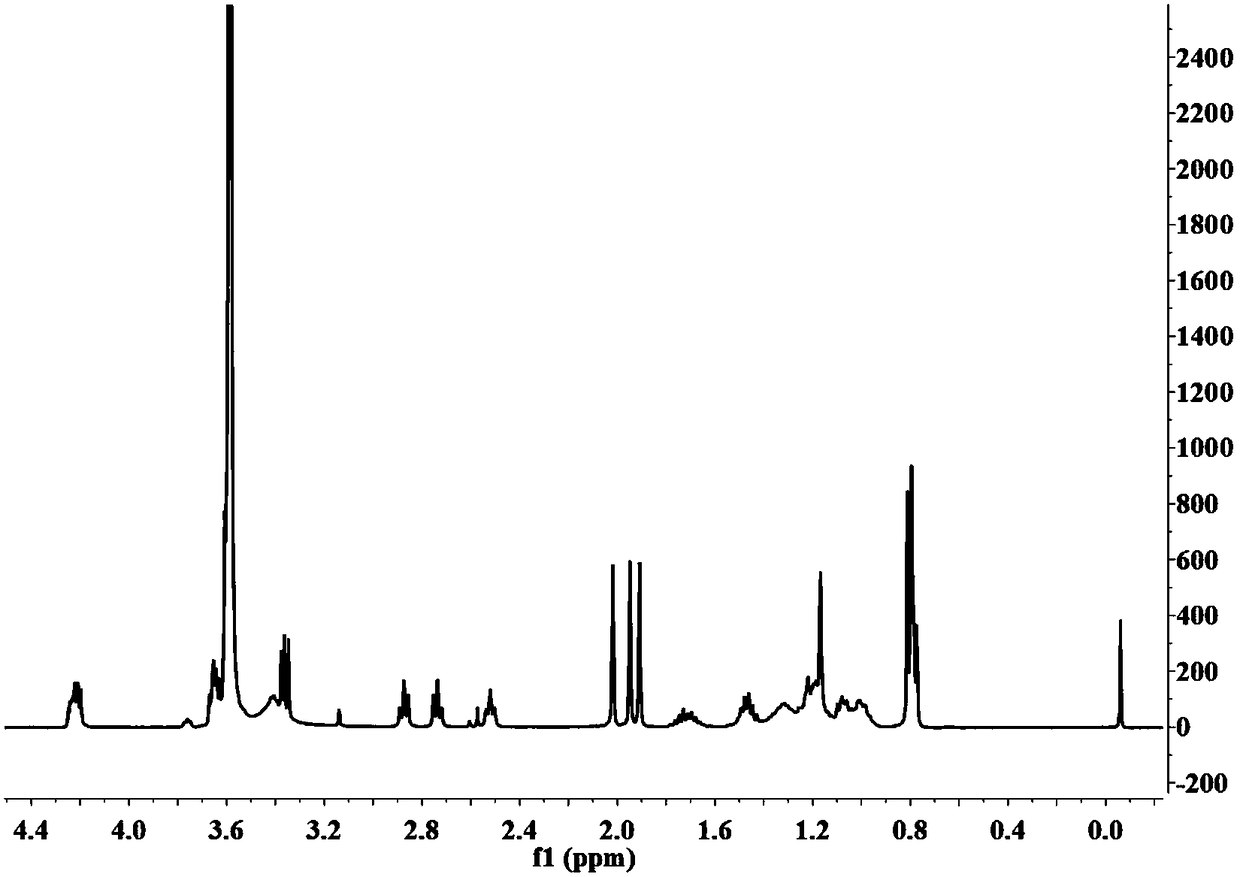
Image
Examples
Embodiment 1
[0052] This embodiment provides a paclitaxel prodrug with both P-glycoprotein inhibitory function and redox dual response characteristics, which is synthesized by the following steps:
[0053] (1) Synthesis of 2,2'-thiodiacetic anhydride: Weigh 3.0g of 2,2'-thiodiacetic acid into a 50mL round bottom flask, add 15mL of acetic anhydride. After reflux reaction at 65°C for 4 hours, acetic anhydride, acetic acid, etc. were distilled off under reduced pressure; an appropriate amount of ether was added, and rotary evaporation was repeated 2-3 times to obtain a white solid powder, which was dried under vacuum at 40°C overnight to obtain 2,2'- Thiodiacetic anhydride, the yield is 98.7%.
[0054] (2) Synthesis of TPGS-S-COOH: Weigh 1.5g TPGS into a 25mL round bottom flask, add 10mL N,N-dimethylformamide to dissolve, then add 0.122g 4-dimethylaminopyridine (DMAP ) and 0.225g 2,2'-thiodiacetic anhydride at 60°C for 12-16h. After the reaction, the reaction solution was placed in a dialys...
Embodiment 2
[0065] This embodiment provides a paclitaxel prodrug with both P-glycoprotein inhibitory function and redox dual response characteristics, which is synthesized by the following steps:
[0066] (1) Synthesis of (ethylenedithio)diacetic anhydride: Weigh 2.0 g of (ethylenedithio)diacetic acid into a 50 mL round bottom flask, and add 20 mL of anhydrous acetyl chloride. After reflux reaction at 65°C for 4 hours, acetyl chloride, acetic acid, etc. were distilled off under reduced pressure; an appropriate amount of ether was added, and the rotary evaporation was repeated 2-3 times to obtain a white solid powder, which was dried in vacuum at 40°C overnight, namely (ethylene di Thio) diacetic anhydride, the yield is 95.6%.
[0067] (2) Synthesis of TPGS-SCCS-COOH: Weigh 1.7g TPGS, 0.122g 4-dimethylaminopyridine and 0.31g (ethylenedithio) diacetic anhydride in a 50mL round bottom flask, add 20mL pyridine After dissolving, react at 60°C for 16h. After the reaction was completed, the py...
Embodiment 3
[0078] This embodiment provides a paclitaxel prodrug with both P-glycoprotein inhibitory function and redox dual response characteristics, which is synthesized by the following steps:
[0079] (1) Synthesis of TPGS-pNC: Weigh 3.0g TPGS into a 50mL round-bottomed flask, dry it in vacuum at 60°C for 4h, then add 10mL anhydrous dichloromethane, anhydrous tetrahydrofuran, anhydrous N,N-dimethylformaldehyde After the amide, anhydrous acetone or anhydrous dimethylsulfoxide are dissolved, add 0.45 mL of anhydrous triethylamine (TEA). Weigh 0.61g of 4-nitrophenyl chloroformate (pNC), 10mL of anhydrous dichloromethane, anhydrous tetrahydrofuran, anhydrous N,N-dimethylformamide, anhydrous acetone or anhydrous dimethylmethylene After the maple was dissolved, it was added dropwise to the TPGS dichloromethane solution under ice cooling. After the dropwise addition, the reaction was continued for 12 h at room temperature. After the reaction, dichloromethane was removed by rotary evaporati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com