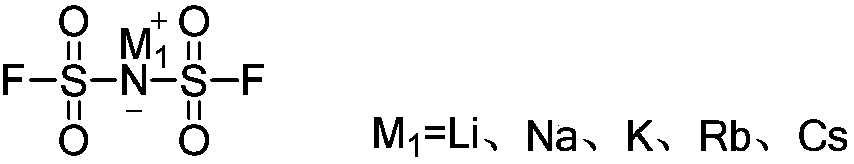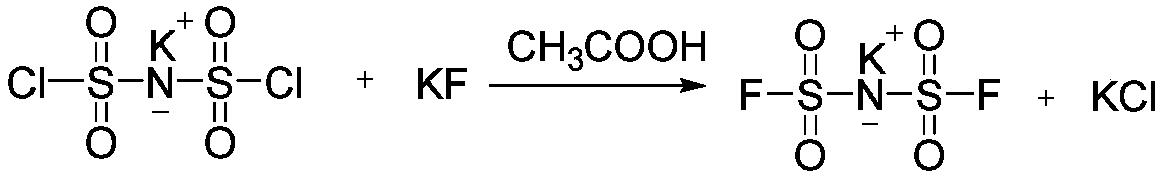Preparation method of bis(fluorosulfonyl)imine alkali metal salt
A technology of imine bases and metal salts, applied in the direction of nitrogen and non-metallic compounds, nitrosyl chloride, etc., can solve problems such as increased difficulty, poor effect of ionic liquids, harsh reaction conditions, etc., to improve quality and performance, and production costs Inexpensive and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
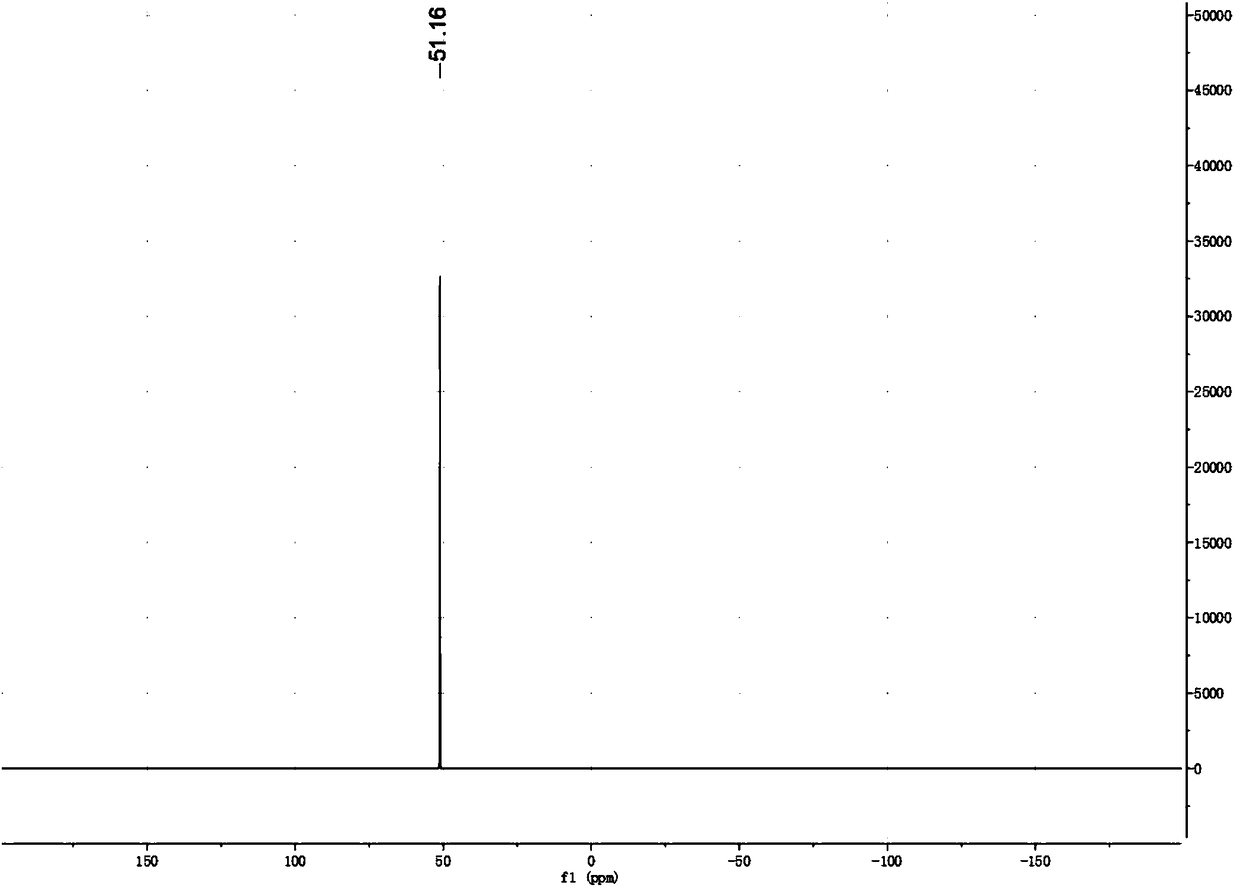
[0028] The synthesis of potassium bis(fluorosulfonyl)imide (KFSI) is as follows:
[0029]
[0030] Under nitrogen protection, KClSI (125.5 g, 0.5 mol), CH 3 COOH (60mL), after stirring at room temperature for 0.5h, KF (58g, 1mol) was added, and after reacting at room temperature for 20h, the protic solvent was evaporated with a rotary evaporator for recycling. Then add 120 mL of organic solvent ethyl acetate to filter the insoluble matter, collect the filtrate to concentrate and recover the solvent, then add 100 mL of ethanol to the concentrated solution, heat and reflux for 2 hours, filter a small amount of insoluble matter while it is hot, concentrate the filtrate, and then add dropwise the poor organic solvent dichloro 80 mL of methane precipitated colorless crystals, then filtered and dried in vacuo to obtain 88 g of colorless crystals with a yield of 80% and a content of ≥99%.
Embodiment 2
[0032] The synthesis of bis(fluorosulfonyl)imide sodium (NaFSI) is as follows:
[0033]
[0034] Under nitrogen protection, NaClSI (116.5 g, 0.5 mol) and HCOOH (20 mL) were successively added to a 500 mL three-neck flask equipped with a stirrer, and NaF (42 g, 1 mol) was added after stirring at room temperature for 0.5 h under stirring, and then After reacting at room temperature for 18 h, the protic solvent was evaporated with a rotary evaporator for recycling. Then add 120mL of organic solvent dimethyl carbonate to filter the insoluble matter, collect the filtrate to concentrate and recover the solvent, then add 100mL of methanol to the concentrated solution, heat and reflux for 2h, filter a small amount of insoluble matter while it is hot, concentrate the filtrate, and then add the poor organic solvent ring Hexane 80mL, colorless crystals were precipitated, filtered, and vacuum-dried to obtain 76g of colorless crystals, yield 76%, content ≥ 99%.
Embodiment 3
[0036] The synthesis of lithium bis(fluorosulfonyl)imide (LiFSI) is as follows:
[0037]
[0038] Under nitrogen protection, LiClSI (109.5 g, 0.5 mol), CH 3COOH (60mL), under stirring, stirred at room temperature for 0.5h, then added LiF (26g, 1mol), then reacted at room temperature for 24h, and evaporated the protic solvent with a rotary evaporator for recycling. Then add 120 mL of organic solvent acetonitrile to filter the insoluble matter, collect the filtrate to concentrate and recover the solvent, then add 100 mL of ethanol to the concentrated liquid, heat to reflux for 2 hours, filter a small amount of insoluble matter while it is hot, concentrate the filtrate, and then add dropwise 80 mL of poor organic solvent dichloromethane , colorless crystals were precipitated, filtered, and vacuum-dried to obtain 70 g of colorless crystals with a yield of 75% and a content of ≥99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com