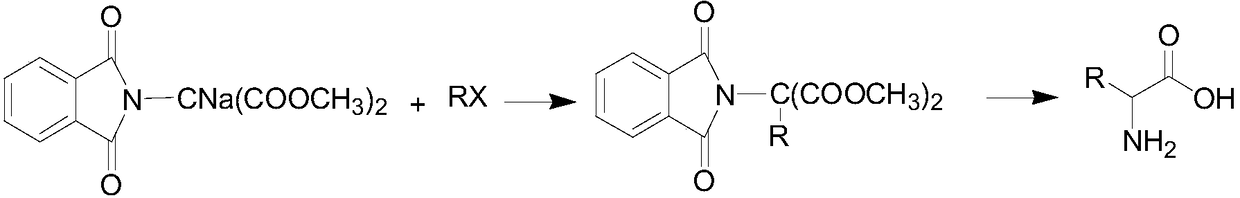
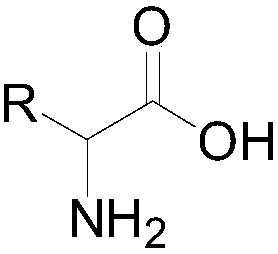
Synthetic method of stable isotope deuterium-labeled alpha-amino acid
A technology of stable isotope and synthesis method, which is applied in the field of synthesis of stable isotope deuterium-labeled α-amino acid, can solve the problems of long reaction steps, dilution of abundance, low utilization rate of isotope atoms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A stable isotope labeled alanine-D 3 The preparation method, this method comprises the following steps:
[0056] 1. Methyl substituted phthalimide dimethyl malonate-D 3 Synthesis
[0057] Add 3.98g of phthalimide dimethyl malonate sodium salt into a 50mL reaction bottle, 20mL of chloroform and stir to disperse, then add iodomethane-D 3 1.65g, the reaction temperature is 40 ℃, and the reaction time is 2 hours. After the reaction is completed, wash with water and dry to obtain 3.68g product. The yield is represented by methyl iodide-D 3 Total 84.1%, HPLC detection, purity 99.5%; mass spectrometry detection, abundance 99.5atom%D.
[0058] 2. Alanine-D 3 Synthesis
[0059] Add methyl substituted phthalimide dimethyl malonate-D in a 50mL reaction flask 31.94g, after 20mL of absolute ethanol was stirred and dissolved, 10mL of sodium hydroxide aqueous solution was added, and the reaction time was refluxed for 4 hours. The yield of base-substituted phthalimide dimethyl ...
Embodiment 2
[0061] A stable isotope labeled valine-D 6 The preparation method, this method comprises the following steps:
[0062] 1. Isopropyl substituted phthalimide dimethyl malonate-D 6 Synthesis
[0063] Add 2.00g of phthalimide dimethyl malonate sodium salt into a 50mL reaction bottle, stir and dissolve DMF20mL, then add bromoisopropane-D 6 1.55g, the reaction temperature is 100°C, and the reaction time is 5 hours. After the reaction is completed, wash with water and dry to obtain 1.70g product. The yield is represented by bromoisopropane-D 6 Total 79.1%, HPLC detection, purity 99.5%; mass spectrometry detection, abundance 99.3atom%D.
[0064] 2. Valine-D 6 Synthesis
[0065] Add isopropyl substituted phthalimide dimethyl malonate-D in a 50mL reaction flask 6 2.15g, after 20mL of anhydrous methanol was stirred and dissolved, 5mL of potassium hydroxide aqueous solution was added, and the reaction time was refluxed for 2 hours. The yield of propyl-substituted phthalimide dimet...
Embodiment 3
[0067] A stable isotope labeled leucine-D 6 The preparation method, this method comprises the following steps:
[0068] 1. Isobutyl substituted phthalimide dimethyl malonate-D 6 Synthesis
[0069] Add 6.00g of phthalimide dimethyl malonate sodium salt into a 50mL reaction bottle, stir and dissolve 15mL of DMSO, then add bromoisobutane-D 6 1.43g, the reaction temperature is 120°C, and the reaction time is 2 hours. After the reaction is completed, it is washed with water and dried to obtain 6.57g of product. The yield is represented by bromoisobutane-D 6 Total 91.3%, HPLC detection, purity 99.4%; mass spectrometry detection, abundance 99.4atom%D.
[0070] 2. Leucine-D 6 Synthesis
[0071] Add isobutyl substituted phthalimide dimethyl malonate-D in a 50mL reaction flask 6 2.40g, after stirring and dissolving in 10mL of absolute ethanol, add 15mL of potassium carbonate aqueous solution, and reflux for 6 hours. The yield of dimethyl malonate substituted with phthalimide was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com