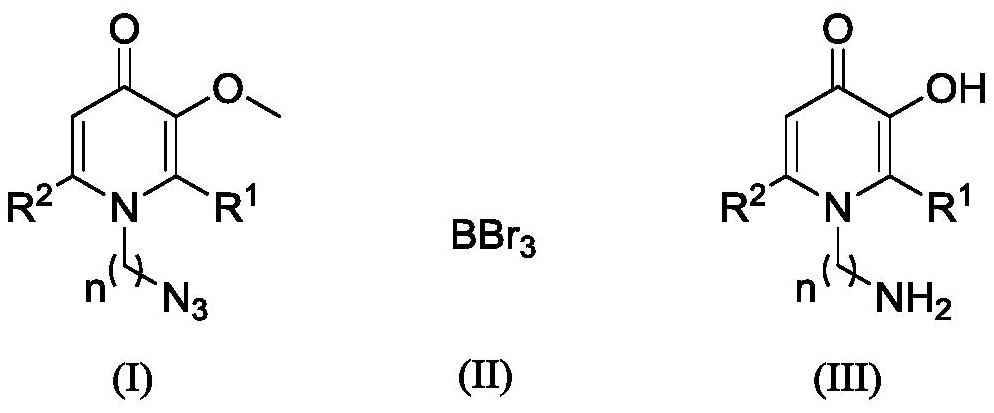
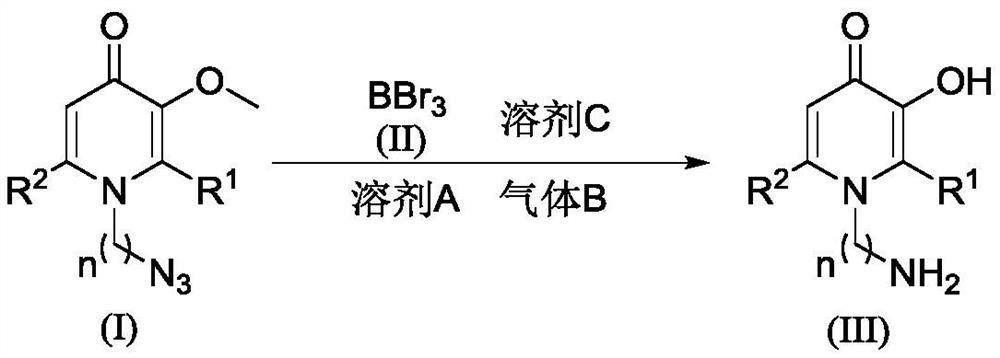
A kind of synthetic method of amino-containing hydroxypyridone compound
A technology for azide hydroxypyridone and ketone compounds, which is applied in the field of synthesis of hydroxypyridone compounds and can solve the problems of harsh reaction conditions, use of metals or toxic reagents, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
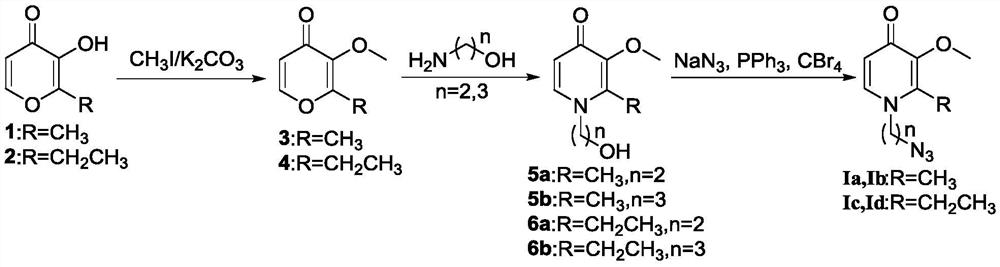
[0065] Embodiment 1: the synthesis of compound 5,6
[0066]
[0067] Add maltol 1 (15.10g, 120mmol) or ethyl maltol 2 (16.80g, 120mmol), methyl iodide (51.10g, 360mmol) into a 500mL single-necked flask, anhydrous K 2 CO 3 (49.70g, 360mmol), acetone (100mL), heated to reflux for 3h. The reaction was monitored by TLC. After the conversion of the raw materials was complete, the reaction was stopped, the reaction solution was concentrated to obtain a solid, 180 mL of water was added, extracted with 180 mL×3 dichloromethane, the organic layers were combined, and the organic layer was washed with 100 mL×2 saturated brine, and anhydrous After drying over sodium sulfate and concentrating, 16.70 g (99.4%) of yellow liquid 3 or 18.40 g (99.5%) of yellow liquid 4 were obtained.
[0068] 3 (7.00g, 50mmol) or 4 (7.70g, 50mmol), ethanolamine (9.15g, 150mmol) or 3-aminopropanol (11.25g, 150mmol), 2M NaOH (5mL), ethanol (100mL ), warming up to reflux reaction 8h. The reaction was monit...
Embodiment 2
[0069] Embodiment 2: raw material Ia, Ib, Ic, the synthesis of Id
[0070]
[0071] Add 5a (3.66g, 20mmol, n=2) or 5b (3.94g, 20mmol, n=3) or 6a (3.94g, 20mmol, n=2) or 6b (4.22g, 20mmol, n=2) or 6b (4.22g, 20mmol, n=2) into a 100mL single-necked flask 3), anhydrous DMF (50mL), sodium azide (6.50g, 100mmol), triphenylphosphine (10.48g, 40mmol), carbon tetrabromide (13.28g, 40mmol) were added successively, and reacted at room temperature for 20min. TLC monitors the reaction, and after the conversion of raw materials is complete, the reaction is stopped, filtered, and the filtrate is concentrated, purified by silica gel column chromatography (dichloromethane:methanol=20:1 as eluent) to obtain 3.26g (78.4%, 78.4%) of yellow oily liquid Ia n=2), Ib 2.93g (66.1%, n=3), Ic 3.15g (71%, n=2), Id 3.71g (78.5%, n=3).
Embodiment 3
[0072] Embodiment 3: the synthesis of compound 12
[0073]
[0074] Add kojic acid 7 (142.00g, 1000mmol) and thionyl chloride (500mL) into a 1000mL single-necked flask, connect the reaction flask to a gas absorption device, and stir at room temperature for 2h. After the reaction, suction filtration was performed, the filter cake was washed with petroleum ether until the filtrate was colorless, and the filter cake was recrystallized with water to obtain 144.50 g (90.3%) of a white solid. Add the above-mentioned white solid (144.50g, 903.13mmol) and distilled water (500mL) into a 1000mL two-necked flask, heat up to 50°C, then add zinc powder (97.5g, 1500mmol), measure concentrated hydrochloric acid (200mL) and place under constant pressure In the liquid funnel, slowly add dropwise, control the temperature between 70 ~ 80 ℃, after dropping, control the temperature at 75 ℃ for 4 hours. After the reaction, the reaction solution was cooled to room temperature, extracted with 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com