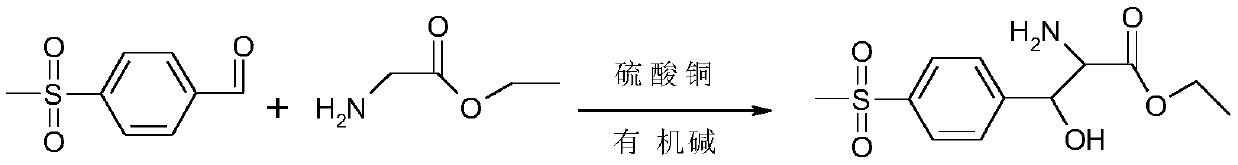
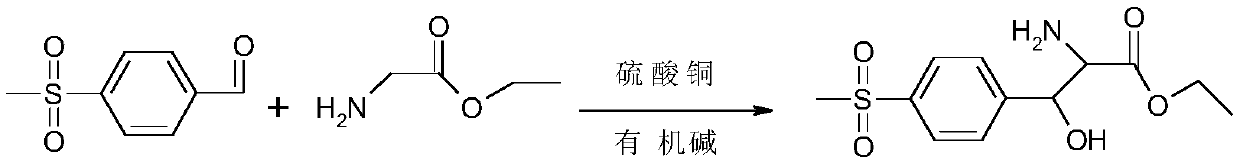
The synthetic method of p-thymphenyl phenyl serine ethyl ester and p-thymphenyl phenyl serine ethyl ester
A technology of thiamphenylphenylserine ethyl ester and glycine ethyl ester hydrochloride, which is applied in the synthesis of p-thymphenylphenylserine ethyl ester and p-thymphenylphenylserine ethyl ester field, which can solve unfavorable esterification Forward progress, unfavorable production rate, cumbersome process and other problems, to achieve the effect of improving reaction efficiency, benefiting environmental protection, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention discloses the synthetic method of p-thymphenyl phenyl serine ethyl ester, comprising the following steps:
[0037] S1, add the set dose of glycine ethyl ester hydrochloride and methanol into the reaction kettle, heat up to 30-80°C, preferably between 40-65°C, combine stirring when heating, and make glycine ethyl ester hydrochloride Dissolve in methanol to form the first solution.
[0038] S2, add the set dose of copper sulfate pentahydrate into the first solution to dissolve, and keep stirring at 30-80°C, preferably at 40-65°C for 0.5h to form the second solution, at this time in the second solution The reaction produces glycine ethyl ester copper complex.
[0039] S3, adding an organic base in the second solution, the organic base is preferably one of triethylamine, dimethylamine, and ethylenediamine, so that the pH value of the second solution is adjusted to alkaline reaction conditions, that is: Make the pH value of the second solution between...
Embodiment 1
[0046] Add 500g (15.6mol) of methanol and 73.9g (0.53mol) of ethyl glycine hydrochloride into a 1000ml reaction flask, stir and heat up to 50-55°C to completely dissolve ethyl glycine hydrochloride in methanol to form the first solution.
[0047] Then add 67.5 g (0.27 mol) of copper sulfate pentahydrate into the first solution, stir to dissolve it completely, and continue to keep stirring at 50-55° C. for 0.5 h to obtain the second solution.
[0048]Next, start to add triethylamine dropwise to the second solution to adjust the pH value of the second solution to 8.5-9.0, then add 92 g (0.5 mol) of p-thiamphenicyl benzaldehyde, and keep the reaction at 50-55 ° C for 30 h , control the pH value between 8.5-9.0 during the heat preservation period, during the reaction, monitor the progress of the reaction by HPLC, and when the percentage of p-thiamphenicyl benzaldehyde calculated by the area normalization method <2%, the reaction is considered to be over .
[0049] Recover methan...
Embodiment 2
[0051] Add 500g (15.6mol) of methanol and 76.7g (0.550mol) of ethyl glycine hydrochloride into a 1000ml reaction flask, stir and heat up to 40-45°C to completely dissolve ethyl glycine hydrochloride to form the first solution.
[0052] Then add 71.2 g (0.285 mol) of copper sulfate pentahydrate into the first solution, stir to dissolve it completely, and continue to keep stirring at 40-45° C. for 0.5 h to obtain the second solution.
[0053] Then start to drop dimethylamine methanol solution in the second solution, the pH value of the second solution is adjusted to between 9.0-9.3, then add p-thiamphenicyl benzaldehyde 92g (0.5mol), and at 40-45 ℃ Insulation reaction 30h, control pH value between 9.0-9.3 during the insulation reaction, during the reaction, monitor the progress of the reaction by HPLC, when the percentage content of p-thiamphenicyl benzaldehyde calculated by the area normalization method<2%, The reaction is considered complete.
[0054] Recover methanol by vacu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com