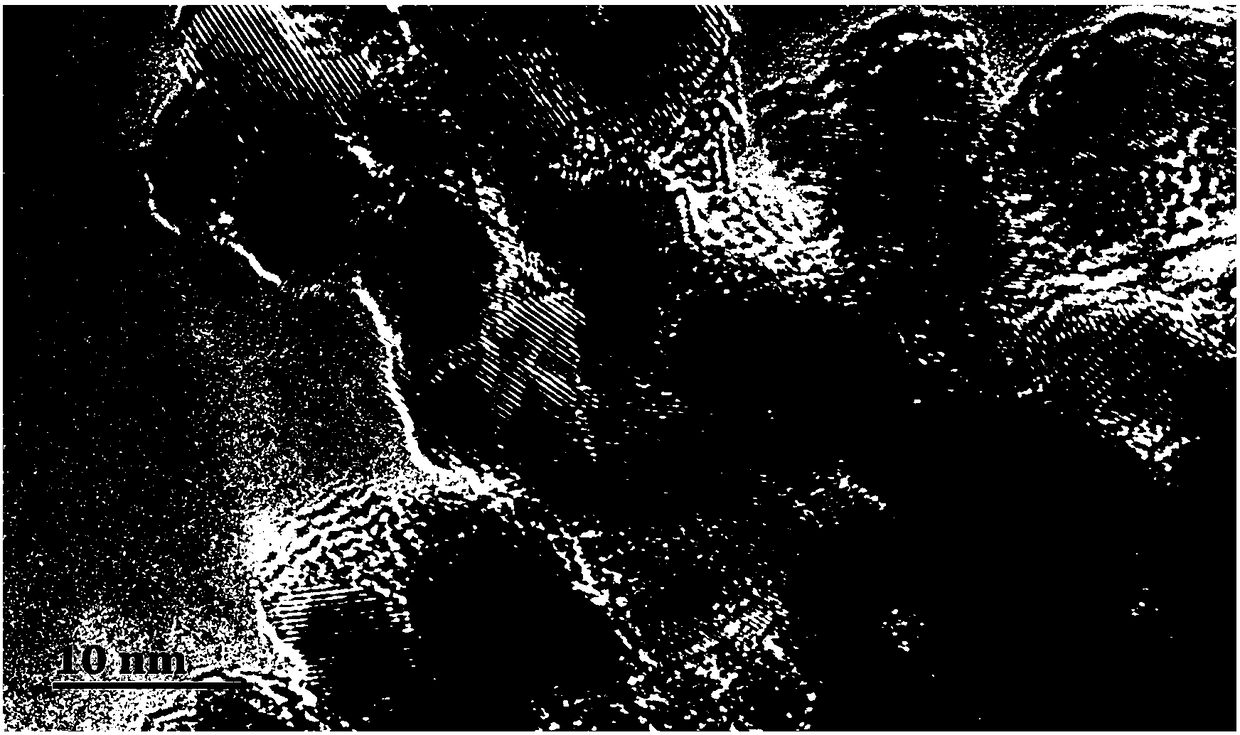
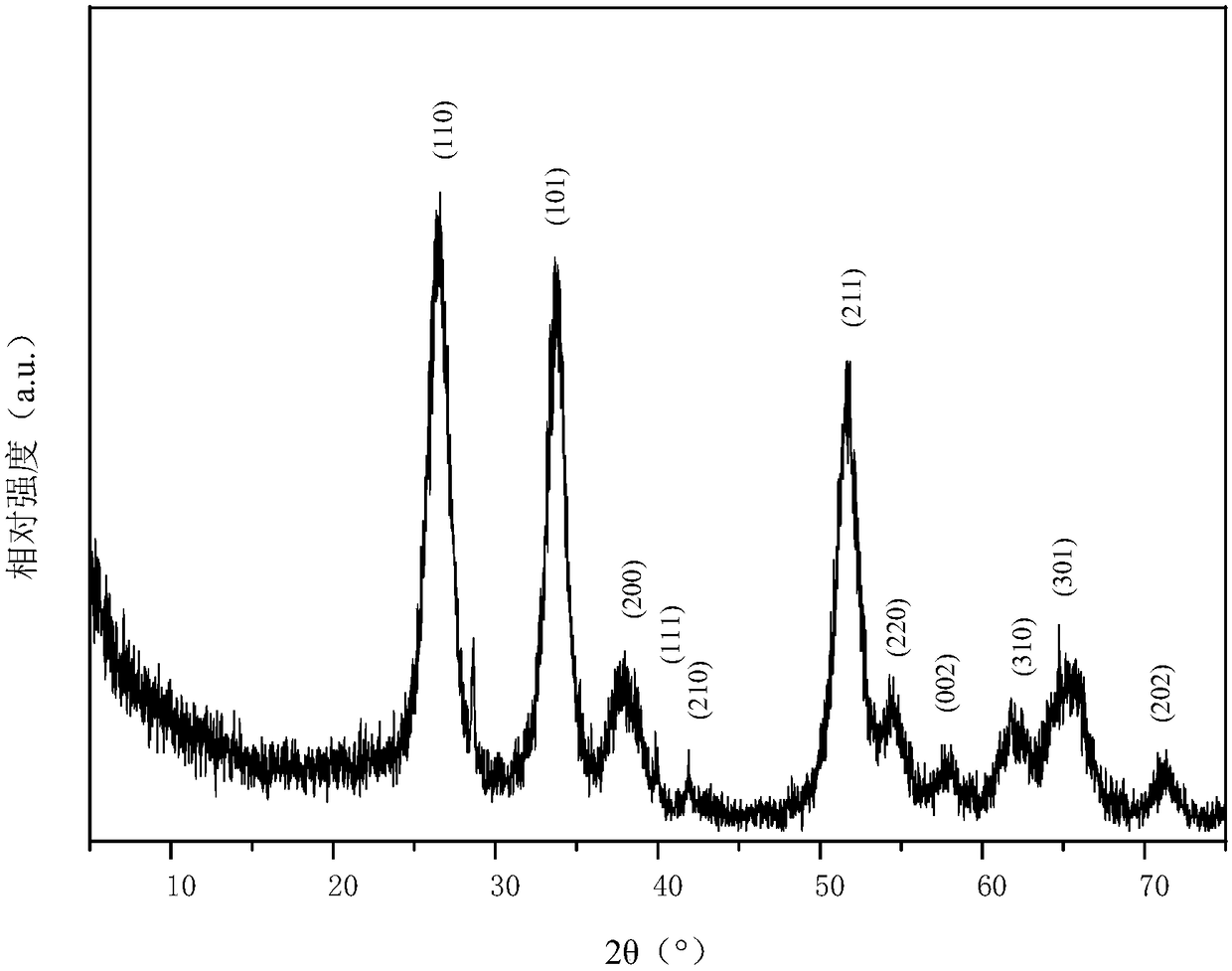
Preparation method of negative electrode material for carbon-coated antimony-doped stannic oxide ion batteries
A tin dioxide, ion battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of low theoretical capacity, poor conductivity, poor cycle stability, etc., to achieve simple material source, increased specific capacity, price low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of carbon-coated antimony-doped tin dioxide ion battery anode material with antimony-tin molar ratio of 6.25%
[0050] Step 1, preparation of antimony-doped tin dioxide;
[0051] tin tetrachloride pentahydrate (SnCl 4 ·5H 2 O) and antimony trichloride (SbCl 3 ) is dissolved in deionized water at a ratio of 6.25% in molar ratio of antimony to tin to obtain solution A; then an appropriate amount of ammonium bicarbonate (NH 4 HCO 3 ) was added to solution A, and after stirring for 10 minutes, solution B was obtained; the resulting solution B was transferred to a 50mL autoclave lined with polytetrafluoroethylene, and kept at a temperature of 180°C for 12 hours, and the precipitate was taken , that is, antimony-doped tin dioxide;
[0052] Dosage: Add 0.71g of tin tetrachloride pentahydrate, 0.073g of antimony trichloride, and 0.32g of ammonium bicarbonate to 20mL of deionized water;
[0053] Step 2, preparation of carbon-coated antimony-doped tin dioxide; ...
Embodiment 2
[0072] Preparation of carbon-coated antimony-doped tin dioxide ion battery anode material with antimony-tin molar ratio of 6%
[0073] Step 1, preparation of antimony-doped tin dioxide;
[0074] tin tetrachloride pentahydrate (SnCl 4 ·5H 2 O) and antimony trichloride (SbCl 3 ) is dissolved in deionized water at a ratio of 6% to antimony-tin ratio to obtain solution A; then ammonium bicarbonate (NH 4 HCO 3 ) was added to solution A, and after stirring for 10 minutes, solution B was obtained; solution B was transferred to a 50mL autoclave lined with polytetrafluoroethylene, and after 15 hours of insulation at a temperature of 160°C, the precipitate was taken. That is, antimony-doped tin dioxide;
[0075] Dosage: Add 0.781g of tin tetrachloride pentahydrate, 0.077g of antimony trichloride, and 0.352g of ammonium bicarbonate to 20mL of deionized water;
[0076] Step 2, preparation of carbon-coated antimony-doped tin dioxide;
[0077] The antimony-doped tin dioxide prepared ...
Embodiment 3
[0092] Preparation of carbon-coated antimony-doped tin dioxide ion battery anode material with antimony-tin molar ratio of 7%
[0093] Step 1, preparation of antimony-doped tin dioxide;
[0094] tin tetrachloride pentahydrate (SnCl 4 ·5H 2 O) and antimony trichloride (SbCl 3 ) is dissolved in deionized water at a ratio of 7% to antimony-tin ratio to obtain solution A; then ammonium bicarbonate (NH 4 HCO 3 ) was added to solution A, and after stirring for 10 minutes, solution B was obtained; the resulting solution B was transferred to a 50mL autoclave lined with polytetrafluoroethylene, and kept at a temperature of 180°C for 8 hours, and the precipitate was taken , that is, antimony-doped tin dioxide;
[0095] Dosage: Add 0.71g of tin tetrachloride pentahydrate, 0.082g of antimony trichloride, and 0.30g of ammonium bicarbonate to 20mL of deionized water;
[0096] Step 2, preparation of carbon-coated antimony-doped tin dioxide;
[0097] The antimony-doped tin dioxide prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com