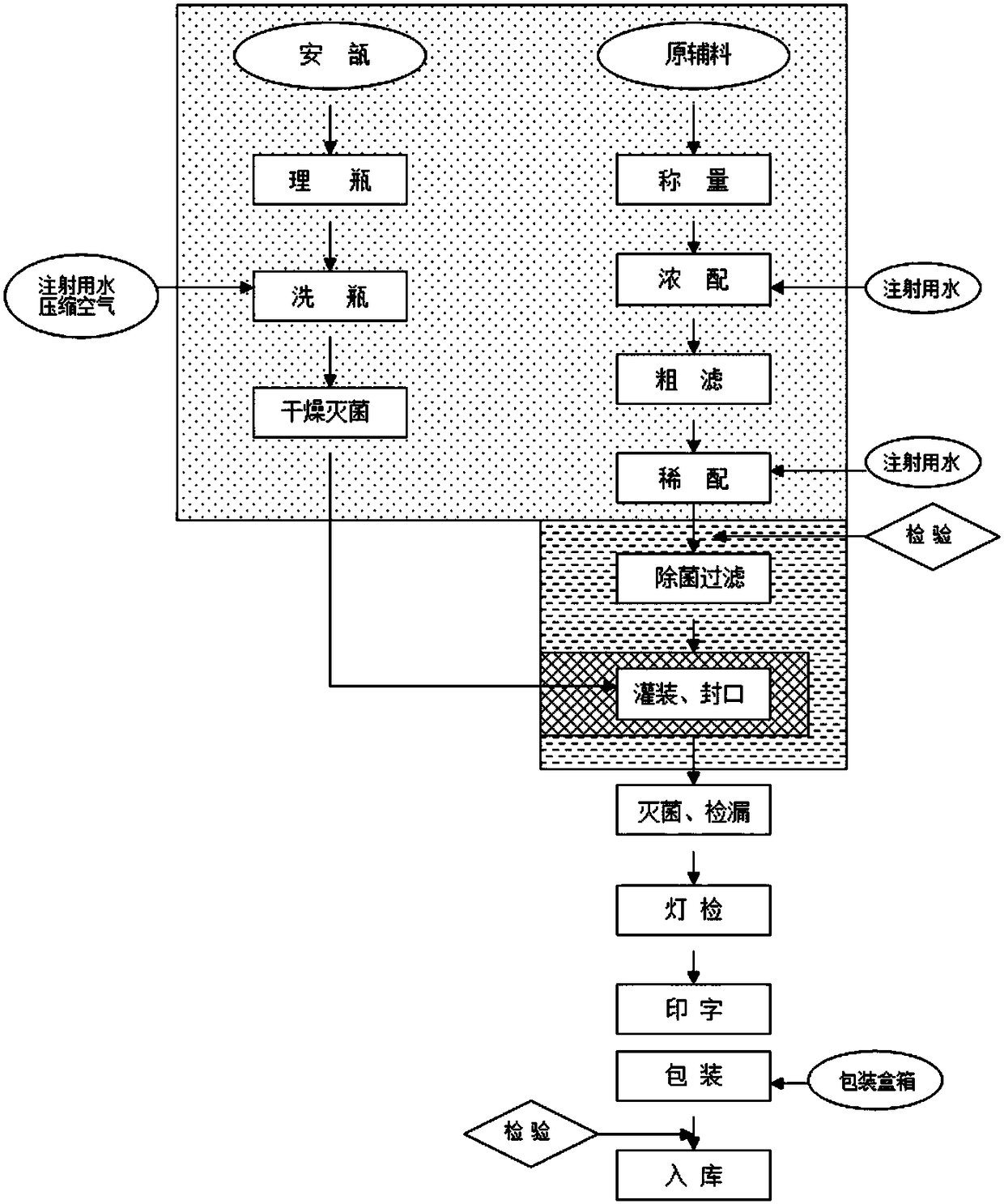
Ginseng glycopeptide injection pharmaceutical composition as well as preparation method and application thereof
A technology of injection and composition, applied in the field of medicine, can solve the problems of patients with cold sweat, general weakness, lack of ginseng glycopeptide hypoglycemic drugs, slow application research, etc., achieves broad market prospects and commercial value, and is suitable for a wide range of people. , the effect of less adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of a ginseng glycopeptide injection pharmaceutical composition, comprising the following steps:
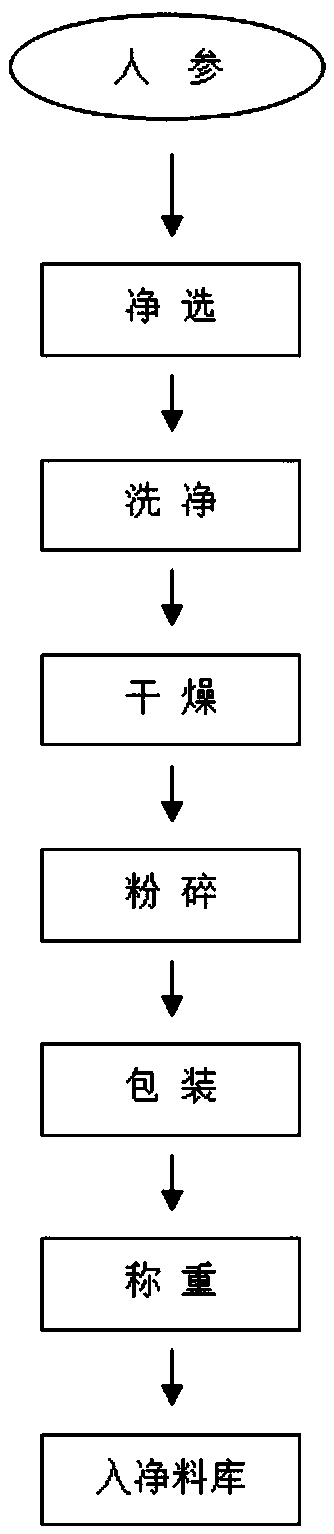
[0046] S1. First remove the non-medicinal parts of the ginseng such as impurities, mildew, insects, and counterfeit products; secondly, wash the ginseng with running water; Under the same conditions, dry for 2-4 hours until the water contained in ginseng is not higher than 12%; finally, use a high-efficiency coarse pulverizer to crush the ginseng into a coarse powder;
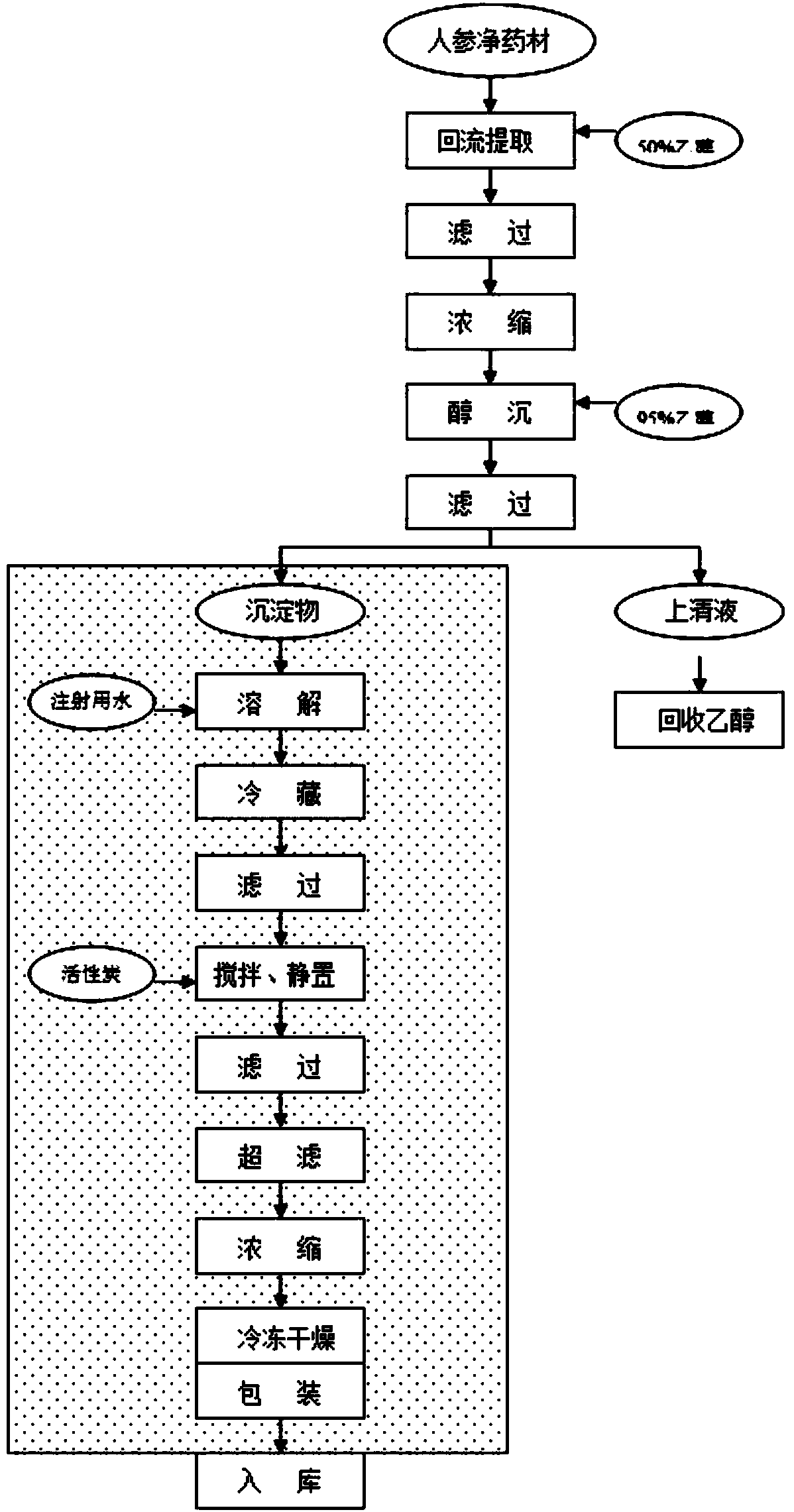
[0047] S2. add 6 times the amount of 50% ethanol to the pretreated ginseng coarse powder for reflux extraction, the extraction time is 2.5 hours, take the filtrate, then use 5.5 times the amount of 50% ethanol to extract the filter residue for 2 hours, take the filtrate, Then use 5.5 times of 50% ethanol to extract the filter residue for 2 hours, remove the filtrate, and combine the three filtrates;
[0048] S3. The three ethanol extracts are concentrated under reduced pressure to a rel...
Embodiment 2
[0059] A preparation method of ginseng glycopeptides, comprising the following steps:
[0060] S1. First remove the non-medicinal parts of the ginseng such as impurities, mildew, insects, and counterfeit products; secondly, wash the ginseng with running water; Under the same conditions, dry for 2-4 hours until the water contained in ginseng is not higher than 12%; finally, use a high-efficiency coarse pulverizer to crush the ginseng into a coarse powder;
[0061] S2. Add 8 times the amount of 50% ethanol to the pretreated ginseng coarse powder for reflux extraction, the extraction time is 3.5 hours, take the filtrate, then use 7.5 times the amount of 50% ethanol to extract the filter residue for 2 hours, take the filtrate, Then use 7.5 times of 50% ethanol to extract the filter residue for 2 hours, remove the filtrate, and combine the three filtrates;
[0062] S3. The three ethanol extracts were concentrated under reduced pressure to a relative density of 1.25.
[0063] S4. ...
Embodiment 3
[0073] A method for preparing ginseng glycopeptides, comprising the following steps:
[0074] S1. First remove the non-medicinal parts of the ginseng such as impurities, mildew, insects, and counterfeit products; secondly, wash the ginseng with running water; Under the same conditions, dry for 2-4 hours until the water contained in ginseng is not higher than 12%; finally, use a high-efficiency coarse pulverizer to crush the ginseng into a coarse powder;
[0075] S2. Add 7 times the amount of 50% ethanol to the pretreated ginseng coarse powder for reflux extraction, the extraction time is 3 hours, take the filtrate, then use 6.5 times the amount of 50% ethanol to extract the filter residue for 2 hours, take the filtrate, Then use 6.5 times of 50% ethanol to extract the filter residue for 2 hours, remove the filtrate, and combine the three filtrates;
[0076] S3. The three ethanol extracts were concentrated under reduced pressure to a relative density of 1.15.
[0077] S4. Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com