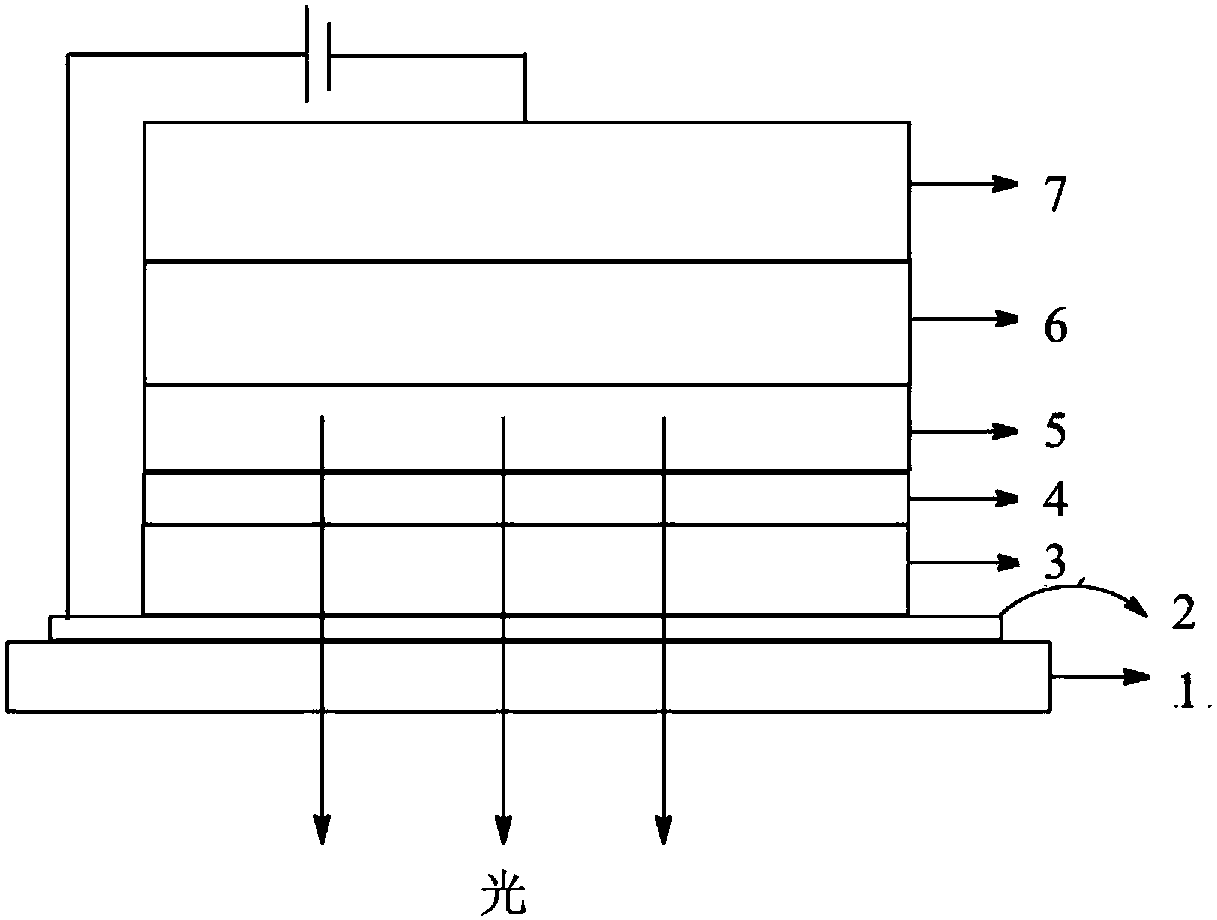
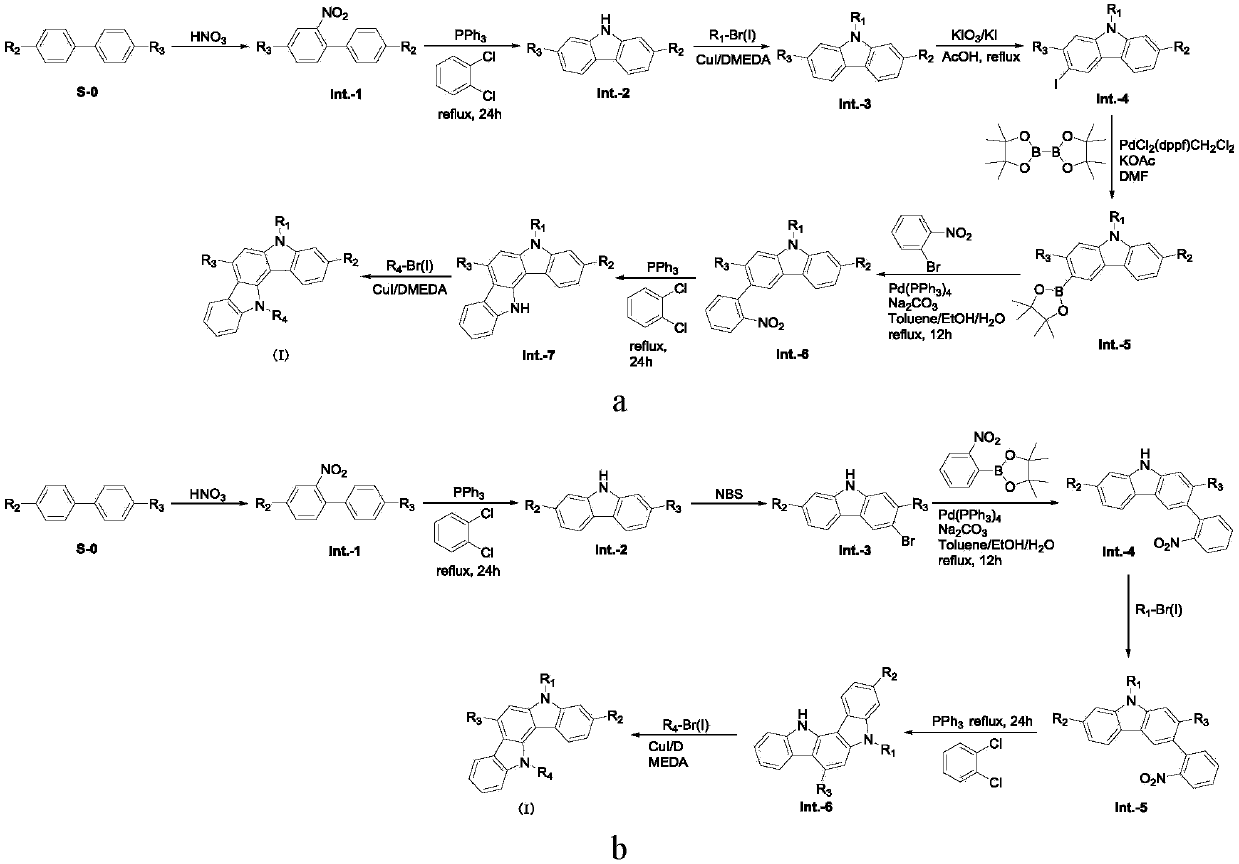
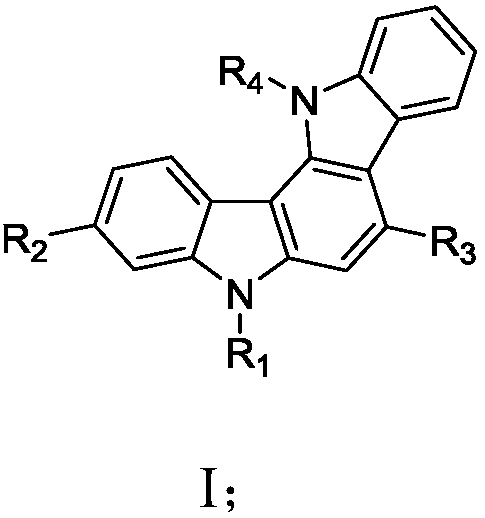
Carbazole derivative and material and organic light-emitting device comprising same
A carbazole derivative, C6-C60 technology, applied in the field of carbazole derivatives, can solve the problems of unbalanced distribution of electrons and holes, uneven molecular weight distribution, and reduced luminous efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The preparation of embodiment 1 compound formula S-1 (synthetic scheme 1)
[0080] The structural formula of compound S-1 is shown in the following formula:
[0081]
[0082] The first step, the preparation of 2-nitro-4,4'-dimethylbiphenyl (Int.-1):
[0083]
[0084] Add 20g of 4,4'-dimethylbiphenyl S-0 in batches to 360ml of 85% nitric acid and 360ml of acetic acid mixed solution, control the temperature and stir at 40°C for 30 minutes, then pour the reaction solution into 2500ml of ice water , extracted three times with dichloromethane, and the organic phase was washed with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated to dryness under reduced pressure, and recrystallized with methanol to obtain 23 g of Int.-1 as a yellow solid, with a yield of 92.5%.
[0085] The second step, the preparation of intermediate Int.-2:
[0086]
[0087] Mix 15g of the first-step intermediate Int.-1 with 100ml of dichlorobenzene, add 86.5g of t...
Embodiment 2
[0112] Embodiment 2, the preparation of compound formula S-8 (synthetic route 2)
[0113] The structural formula of compound S-8 is shown in the following formula:
[0114]
[0115] The first step, the preparation of intermediate Int.-8:
[0116]
[0117] 15g of the intermediate Int.-2 prepared in the second step of Example 1 was dissolved in 150ml of dry N,N-dimethylformamide, and under nitrogen protection, the temperature was cooled to 0°C with an ice-water bath, and 3.4g of NBS was stirred and reacted for 2 hours, then poured into ice water, filtered, the filter cake was washed with water, separated and purified by silica gel column to obtain 19.0 g of intermediate Int.-8, a white solid, with a yield of 90%.
[0118] The second step, the preparation of intermediate Int.-9:
[0119]
[0120] The first step intermediate Int.-8 of 10.0g is dissolved in the tetrahydrofuran of 80ml, under nitrogen protection, add the anhydrous sodium carbonate of 11.0g SM020 and 15.5g...
Embodiment 3
[0136] Embodiment 3, the preparation of compound formula S-25
[0137] The structural formula of compound S-25 is shown in the following formula:
[0138]
[0139] The first step, the preparation of intermediate Int.-12:
[0140]
[0141] 5.0 g of the seventh step product Int.-7 of Example 1, 2.3 g of raw material SM050, 4.0 g of sodium tert-butoxide, 42 mg of cuprous iodide and 60 ml of dry xylene were mixed, and deoxidized under vacuum for 10 minutes , under the protection of nitrogen, add 0.1ml of 10% tri-tert-butylphosphonium toluene solution, heat up and reflux for 24 hours, cool to room temperature, add 50ml of water to the reaction solution for dilution, separate the organic phase, and concentrate to dryness under reduced pressure. The residue was separated and purified by silica gel column to obtain 4.9 g of the intermediate formula Int.-12 as a yellow solid with a yield of 79%.
[0142] Second step, the preparation of compound formula S-25:
[0143]
[014...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com