Vanadium boron co-doped negative electrode material for fuel cell and preparation method thereof
A cathode material and fuel cell technology, applied to battery electrodes, circuits, electrical components, etc., can solve the problems of accelerating the interface reaction between electrodes and electrolytes, making it difficult to effectively improve ionic conductivity, increasing the polarization resistance of battery interfaces, etc., to achieve ionic Good migration ability and stability, improve migration ability, and reduce the effect of internal polarization resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
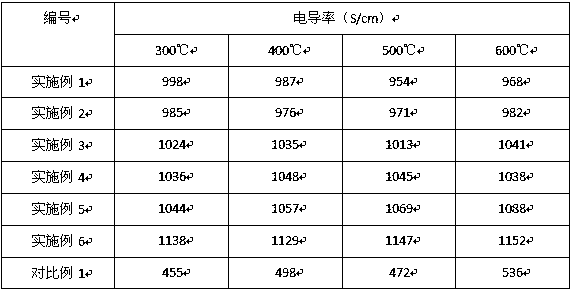
Image
Examples
Embodiment 1
[0041] The vanadium-boron co-doped cathode material for fuel cells is prepared by the following method:
[0042] a. Weighing: Weigh the oxides of metal A and metal B to ensure that the molar ratio of A to B is 1:0.9, where A is La and B is Si;
[0043] b. Preparation of ceramic precursor: After mixing the oxide of metal A and metal B, add vanadium pentoxide and additives, mix and ball mill to make the particle size less than 50 microns, and then in air atmosphere at 300°C Lower calcining 8h, obtain the ceramic precursor that contains a large amount of pores; Wherein, the addition amount of vanadium pentoxide is 10% of the oxide weight sum of the oxide of metal A and metal B; The addition amount of additive is vanadium pentoxide 30% by weight; the auxiliary agent is starch.
[0044] c. Tablet pressing: put the ceramic precursor into a tablet press, and press it under 200MPa to obtain a sheet material;
[0045] d. Low-temperature plasma sintering: Plasma sintering the sheet-sh...
Embodiment 2
[0049] The vanadium-boron co-doped cathode material for fuel cells is prepared by the following method:
[0050] a. Weighing: Weigh the oxides of metal A and metal B to ensure that the molar ratio of A to B is 1:1, where A is Sr and B is Al;
[0051] b. Preparation of ceramic precursor: After mixing the oxide of metal A and metal B, add vanadium pentoxide and additives, mix and ball mill to make the particle size less than 50 microns, and then in air atmosphere at 500 ° C Lower calcining 4h, obtain the ceramic precursor that contains a large amount of pores; Wherein, the addition amount of vanadium pentoxide is 20% of the oxide weight sum of the oxide of metal A and metal B; 50% by weight; the auxiliary agent is polyvinyl alcohol.
[0052] c. Tablet pressing: put the ceramic precursor into a tablet press, and press it under 400MPa to obtain a sheet material;
[0053] d. Low-temperature plasma sintering: Plasma sintering the sheet-shaped material under a mixed gas source of h...
Embodiment 3
[0057] The vanadium-boron co-doped cathode material for fuel cells is prepared by the following method:
[0058] a. Weighing: Weigh the oxides of metal A and metal B to ensure that the molar ratio of A to B is 1:0.95, where A is La and B is Co;
[0059] b. Preparation of ceramic precursor: After mixing the oxide of metal A and metal B, add vanadium pentoxide and additives, mix and ball mill to make the particle size less than 50 microns, and then in air atmosphere at 400 ° C Lower calcination for 5h, to obtain a ceramic precursor containing a large number of pores; wherein, the addition of vanadium pentoxide is 13% of the weight sum of the oxide of metal A and metal B; the addition of the auxiliary agent is 33% by weight; the auxiliary agent is ethyl cellulose.
[0060] c. Tablet pressing: put the ceramic precursor into a tablet press, and press it at 250MPa to obtain a sheet material;
[0061] d. Low-temperature plasma sintering: Plasma sintering the sheet-shaped material u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com