A method for controllable preparation of sodium manganese fluorophosphate cathode material
A technology for preparing sodium manganese fluorophosphate and obtaining sodium manganese fluorophosphate, applied in battery electrodes, structural parts, electrical components, etc., can solve the unreported Na2MnPO morphology controllable preparation technology, the particles are not suitable for battery materials, and unreported Electrochemical performance and other issues, to achieve the effect of improving electrochemical performance, increasing synthesis efficiency, and good crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
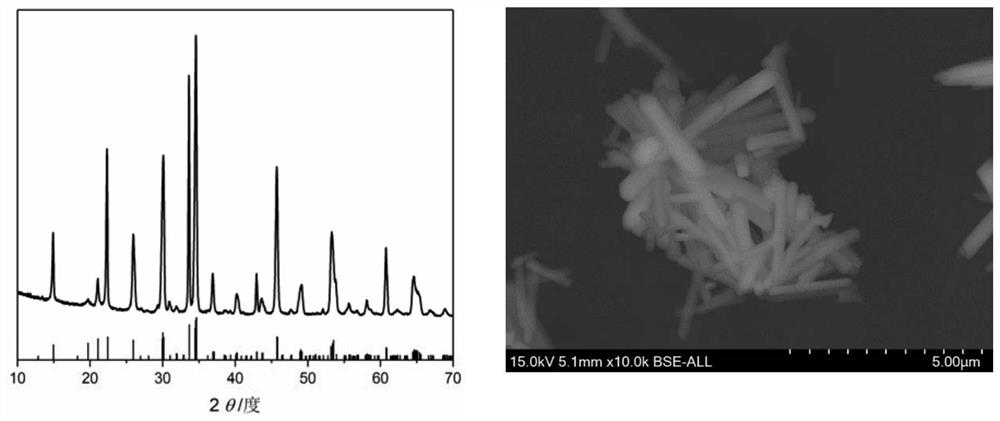
Embodiment 1
[0031] a. Molar ratio Na:Mn:PO 4 : F=6.5: 1: 1: 2.5 Weigh sodium fluoride, sodium hydroxide, phosphoric acid and manganese nitrate, take deionized water as solvent, stir at room temperature, then add ascorbic acid (mass ratio is sodium manganese fluorophosphate: Ascorbic acid=2:1), continue stirring to form a solution or suspension;
[0032]b. Seal the solution or suspension prepared in step a in an autoclave, place it in a blast drying oven at 280°C for 6 hours, and then cool it naturally to room temperature;
[0033] c. Take out the product in the reaction kettle, separate the solid from the liquid, collect the solid, wash it three times with deionized water, then dry it in a blast drying oven at 80°C, and cool it to room temperature to obtain Na 2 PPML 4 F cathode material.
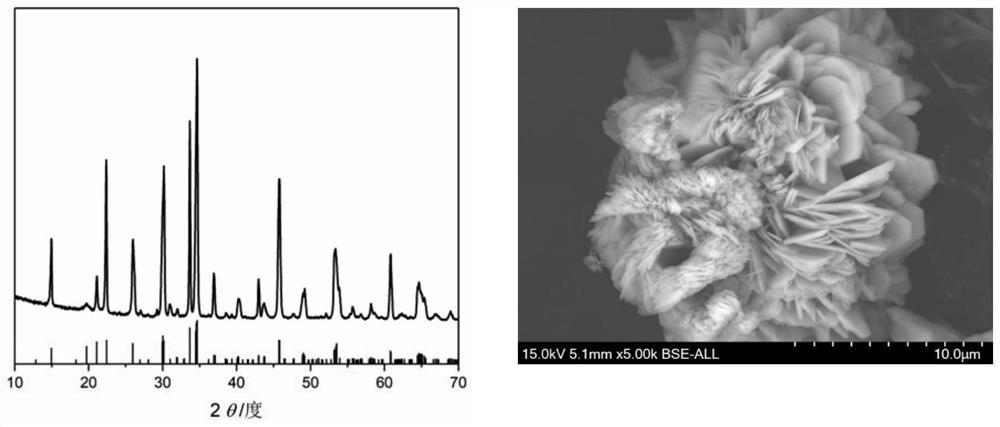
Embodiment 2
[0035] a. Molar ratio Na:Mn:PO 4 : F=6.5: 1: 1: 2.5 Weigh sodium hydroxide, sodium dihydrogen phosphate, manganese nitrate and ammonium fluoride, take deionized water as solvent, stir at room temperature, then add tartaric acid (mass ratio is fluorophosphoric acid Sodium manganese: tartaric acid=2: 0.9), continue stirring to form a solution or suspension;
[0036] b. Seal the solution or suspension prepared in step a in an autoclave, place it in a blast drying oven at 220°C for 12 hours, and then cool it naturally to room temperature;
[0037] c. Take out the product in the reaction kettle, separate the solid from the liquid, collect the solid, wash it three times with deionized water, then dry it in a blast drying oven at 60°C, and cool it to room temperature to obtain Na 2 PPML 4 F cathode material.
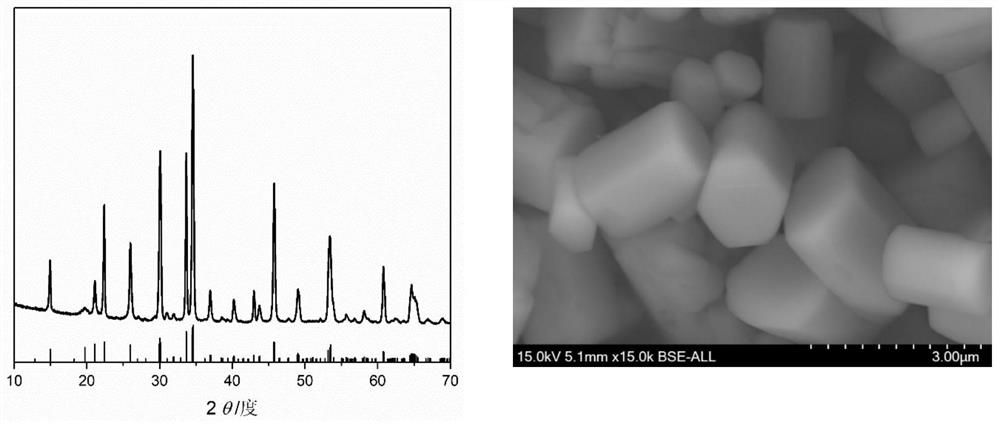
Embodiment 3
[0039] a. Molar ratio Na:Mn:PO 4 : F=6.5: 1: 1: 2.5 Weigh sodium fluoride, sodium hydroxide, ammonium dihydrogen phosphate and manganese acetate, take deionized water as solvent, stir at room temperature, then add citric acid (mass ratio is fluorine Sodium manganese phosphate: citric acid=2: 1), continue stirring to form a solution or suspension;
[0040] b. Seal the solution or suspension prepared in step a in an autoclave, place it in a blast drying oven at 180°C for 72 hours, and then cool it naturally to room temperature;
[0041] c. Take out the product in the reaction kettle, separate the solid from the liquid, collect the solid, wash it three times with deionized water, then dry it in a blast drying oven at 100°C, and cool it to room temperature to obtain Na 2 PPML 4 F cathode material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com