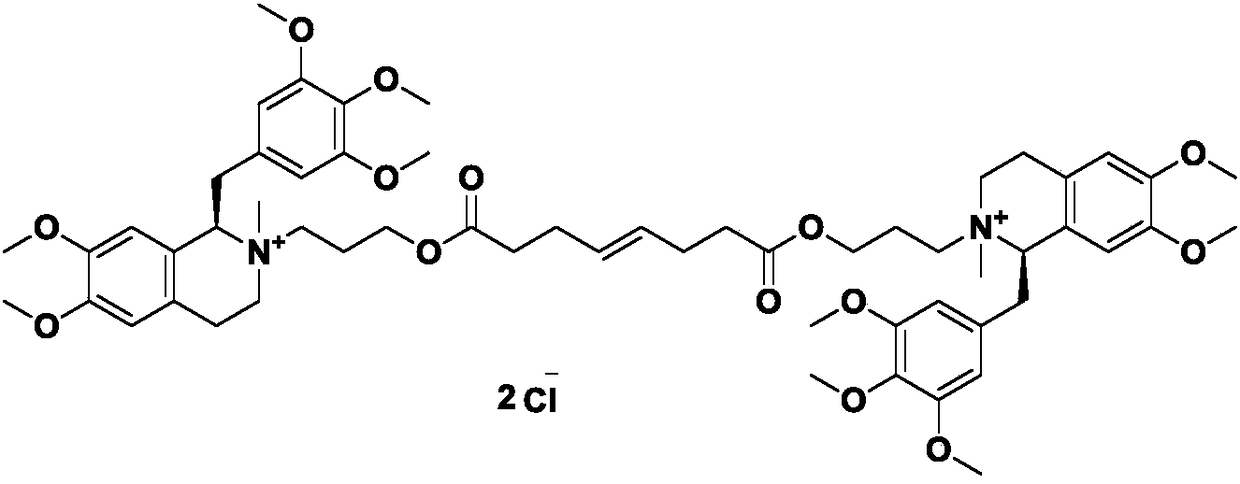
Mivacurium chloride intermediate and method for synthesizing Mivacurium Chloride using mivacurium chloride intermediate
A technology for Micuronium chloride and an intermediate is applied in the field of Micuronium chloride intermediate and synthesizing Micuronium chloride, which can solve the problems of easy moisture absorption and deterioration, complicated and complicated processing and purification, residual and the like, and achieves simple reaction and operation, The effect of green process and stable starting material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The preparation method of 3-hydroxypropyl p-toluenesulfonate is as follows:
[0049]
[0050] Add 100mL triethylamine to 76g (1.0mol) 1,3-propanediol and 150mL dichloromethane, N 2 Cool to 0°C under protection, add 95 g (0.50 mol) of p-toluenesulfonyl chloride dropwise to the above solution, and stir overnight at room temperature. Wash with 150mL of water and 150mL of 1mol / L dilute hydrochloric acid. After completion, the organic phase was anhydrous Na 2 SO 4 After drying, the crude product was spin-dried to obtain the crude product, which was directly put into the reaction when used.
Embodiment 1
[0051] A kind of synthetic method of mivacurium chloride of embodiment 1, comprises the steps:
[0052] Step 1: Condensation Reaction
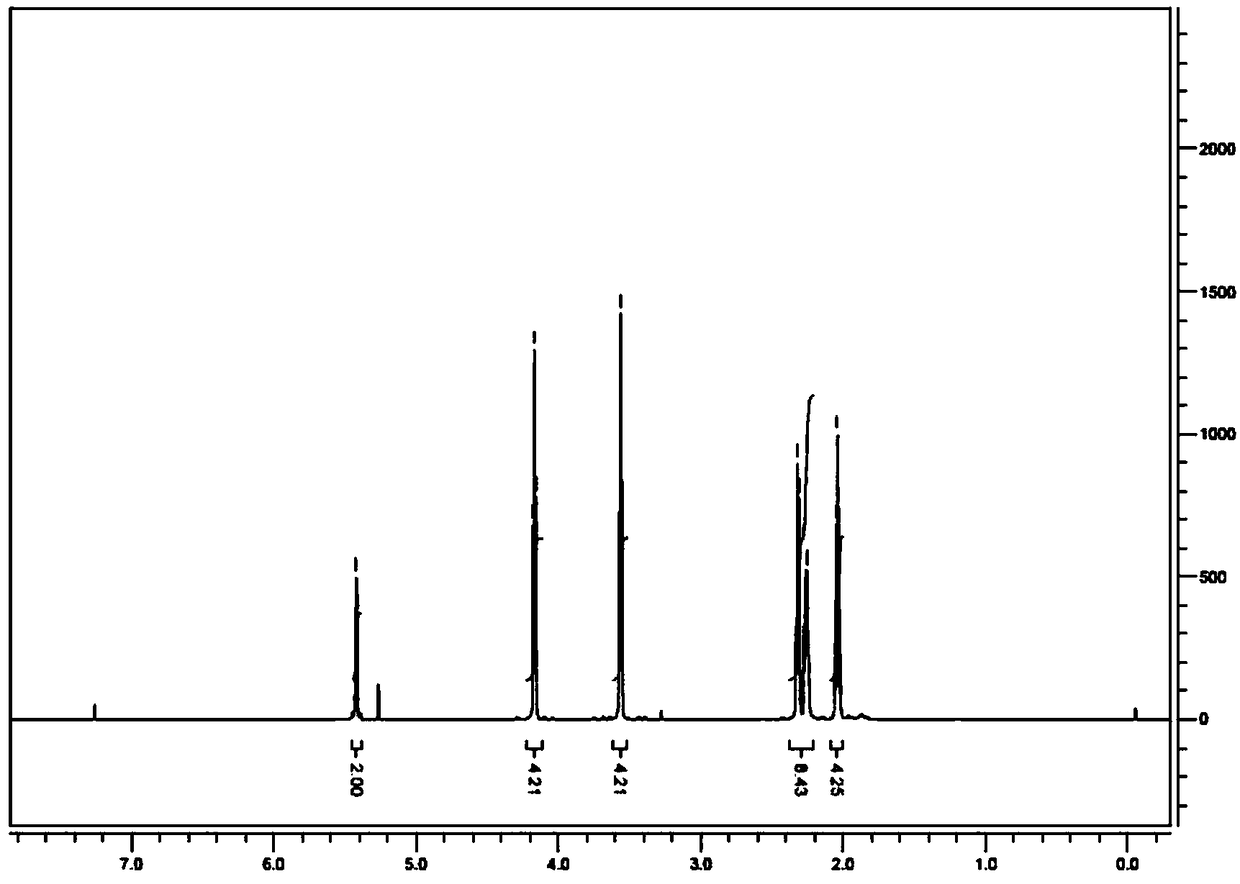
[0053] Add 5.0g of E-oct-4-ene-1,8-dioic acid into 25mL of thionyl chloride, heat and reflux for 3 hours, cool to room temperature after the reaction is completed, spin dry and dissolve with 50mL of dichloromethane, and then Slowly add dropwise at around ℃ into 60mL dichloromethane dissolved with 5.6g 3-chloropropanol, and react at 25℃ for 10 hours after the dropwise addition is completed. After the reaction was completed, 50mL of 1M sodium hydroxide was added to quench the reaction. After separation, the organic phase was dried with anhydrous sodium sulfate and spin-dried to obtain the compound E-oct-4-ene-1,8-dioic acid di- 3-chloropropyl ester 8.3g (yield 86%) light yellow oily liquid, purity 95.6%.
[0054] HNMR (CDCl 3 )δ5.41(2H,m),4.16(4H,m),3.56(4H,m),2.31(4H,m),2.25(4H,m),2.03(4H,m).
[0055] MS (ESI) m / z 326.2.3 (M+1).
[0056] S...
Embodiment 2
[0067] A kind of synthetic method of mivacurium chloride of embodiment 2, comprises the steps:
[0068] Step 1: Condensation Reaction
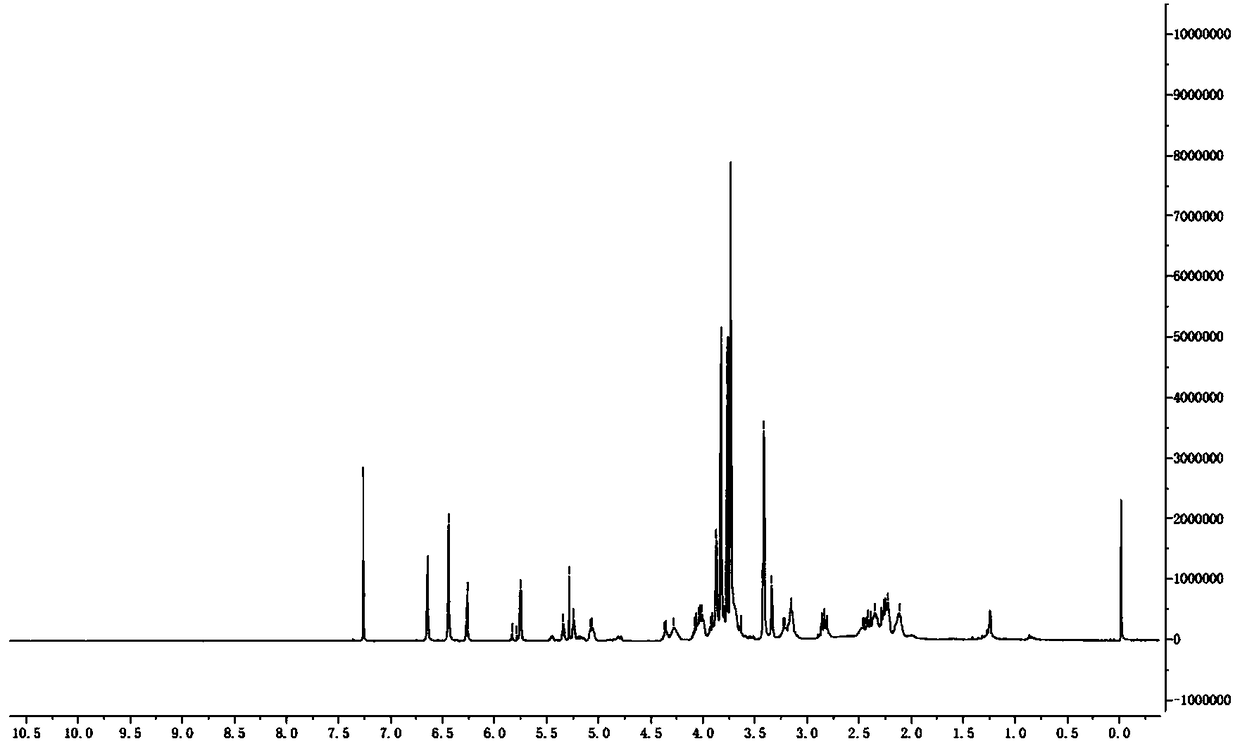
[0069] Add 5g of E-oct-4-ene-1,8-dioic acid into 25mL of thionyl chloride, heat to reflux for 3 hours, cool to room temperature after the reaction is completed, spin dry, dissolve with 50mL of dichloromethane, and then Slowly add it dropwise to 100mL tetrahydrofuran dissolved with 33.5g 3-iodopropanol, and react at 0°C for 24 hours after the dropwise addition is completed. HPLC detected that the reaction was complete, and 50 mL of 1M sodium hydroxide was added to quench the reaction. After liquid separation, the organic phase was dried over anhydrous sodium sulfate. Spin-dry to obtain 12.1 g (yield 81.6%) of compound E-oct-4-ene-1,8-dioic acid di-3-iodopropyl ester, a light yellow oily liquid with a purity of 95.3%.
[0070] HNMR (CDCl 3 )δ5.41(2H,m),4.11(4H,m),3.19(4H,m),2.34(4H,m),2.28(4H,m),2.10(4H,m)
[0071] MS (ESI) m / z 509.2 (M+1)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com