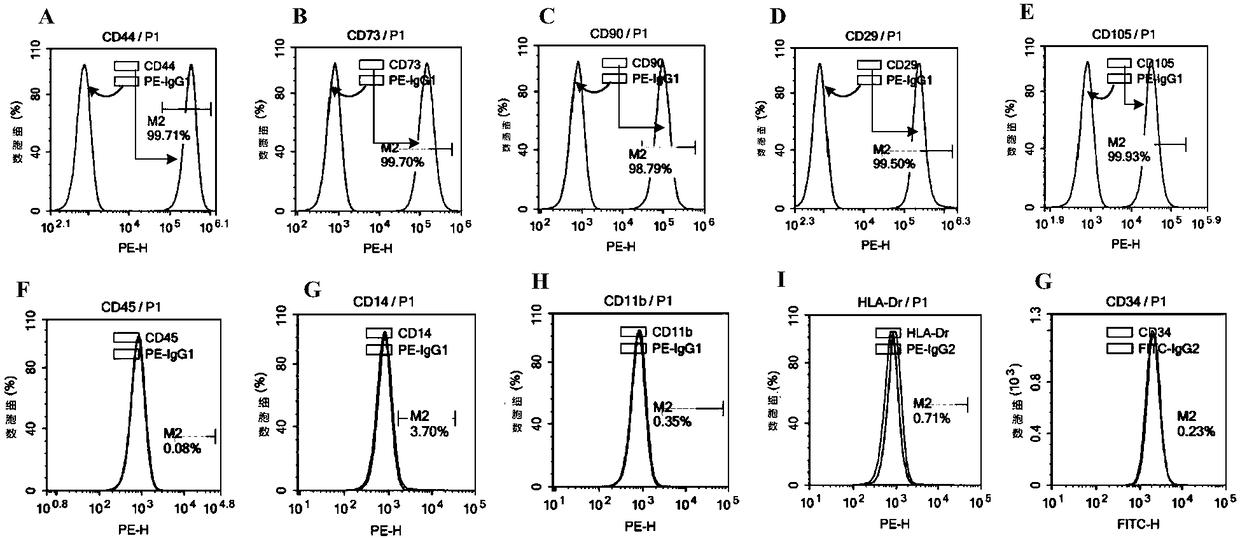
Method for culturing human umbilical cord mesenchymal stem cells
A technology of stem cells and culture methods, applied in cell dissociation methods, cell culture active agents, animal cells, etc., can solve problems such as cell damage, introduction of bovine-derived and pig-derived viruses, easy residual bovine serum albumin, etc., to achieve Broad clinical application prospects, the effect of stable cell properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Primary isolation and culture of HUMSC
[0055] The umbilical cords of healthy fetuses delivered by cesarean section at term were collected. Before the umbilical cord is collected, the puerpera needs to be tested for HIV antibody, hepatitis B virus antibody, hepatitis C virus antibody, Treponema pallidum antibody, etc., all of which are qualified to ensure safety before collection.
[0056] (1) Sterile environment Take an umbilical cord with a length of about 10-15 cm, rinse it repeatedly with sterilized PBS buffer, cut it into 2-3 cm long pieces, and place it in a basal medium (plus 1 % penicillin and streptomycin) in a Petri dish.
[0057] (2) Remove blood vessels, and wash in culture medium until no obvious blood stains.
[0058] (3) In a small amount of culture medium, cut the umbilical cord tissue into small pieces, and use ophthalmic scissors to cut them into pieces of about 1 mm×1 mm.
[0059] (4) Move the fragments to a 50mL centrifuge tube, add 0.3...
Embodiment 2
[0062] Example 2 Subculture of HUMSC
[0063] The specific operation process is as follows:
[0064] (1) When the P0 generation cells in Example 1 are to be fused to 80% to 90%, they are digested with trypsin substitute and centrifuged to remove the supernatant;
[0065] (2) Resuspend the cell pellet with serum-free medium (containing 10% of the total amount of cytokines and chemokines), reseet it in a new culture dish at a ratio of 1:3, and store it in 5% CO 2 , Continue culturing in a 37°C incubator, and the cells are at the P1 generation;
[0066] (3) When the confluence of the P1 generation cells reaches 80-90%, repeat the above operations (1) and (2) to obtain the P2 generation cells.
[0067] The reason for using a petri dish here: the purity of the P0-P2 generation cells gradually increases, and the cell surface markers at the P2 generation are consistent with the detection standards.
Embodiment 3
[0068] Example 3 HUMSC expanded culture
[0069] After the P2 generation cells obtained in Example 2 were digested, they were inoculated in spinner bottles for adherent culture.
[0070] (1) When the P2 generation cells in Example 2 are fused to 80-90%, digest with trypsin substitute, obtain a single cell suspension and centrifuge to remove the supernatant;
[0071] (2) resuspend the cell pellet with serum-free medium (containing 10% of the total amount of cytokines and chemokines);
[0072] (3) After counting the cells, they were reseeded in a spinner bottle and placed in 5% CO 2 , Continue culturing in a 37°C incubator until the confluence of the cells reaches 80-90%, and obtain P3 generation cells;
[0073] (4) Steps (1) (2) (3) above were repeated until the P5 generation of human umbilical cord mesenchymal stem cells was obtained.
[0074] After the cell pellet was resuspended, counted and re-inoculated into a new spinner bottle, the P5 generation cells were obtained by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com