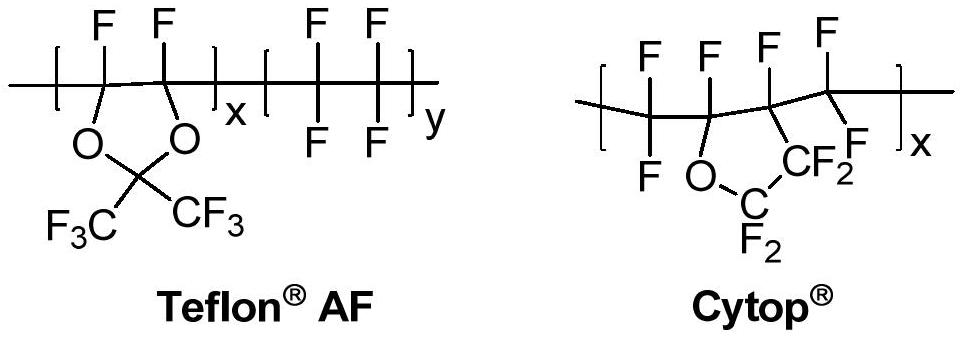
A kind of amorphous fluoropolymer and its preparation method
A polymer and amorphous technology, applied in the direction of organic chemistry, can solve the problems of inability to effectively control material properties, limit the large-scale industrialization and wide application of perfluorinated amorphous polymer materials, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The present invention also provides a method for preparing the above-mentioned amorphous fluoropolymer, comprising:
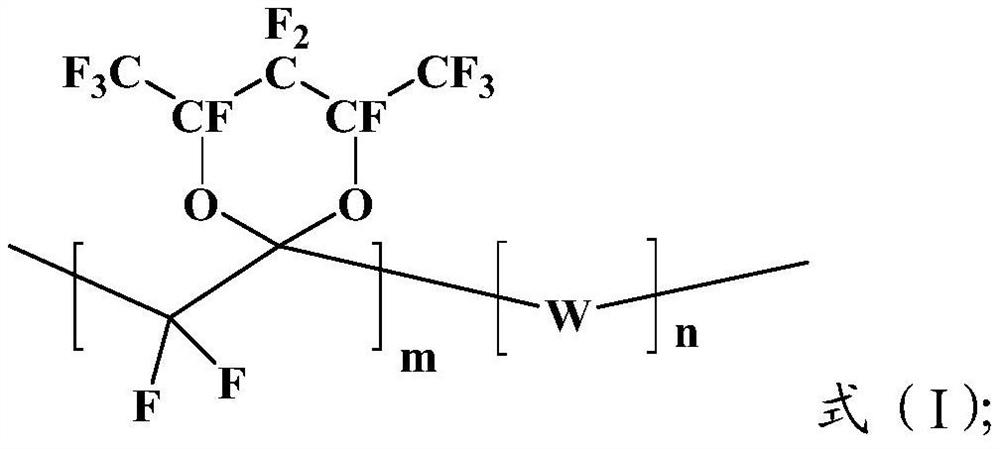
[0046] The fluorine-containing monomer shown in the formula (2) is polymerized with the fluorine-containing olefin in the initiator to obtain the amorphous fluorine-containing polymer shown in the formula (I);
[0047]
[0048] The present invention has no special limitation on the sources of all raw materials, which can be commercially available or self-made.
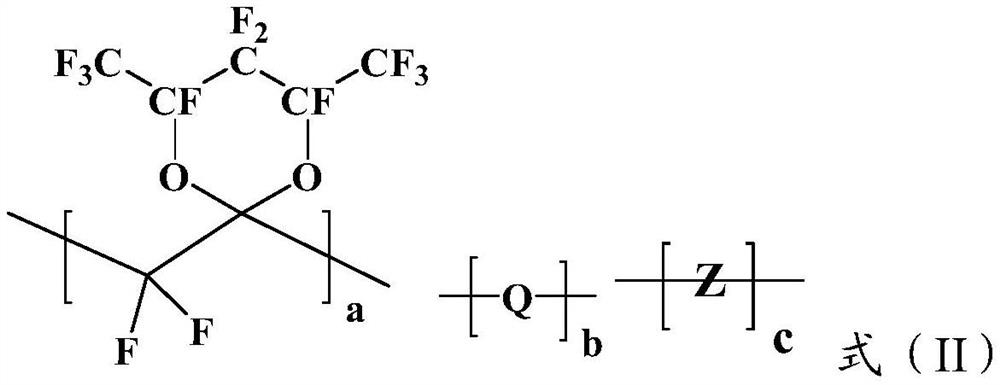
[0049] Wherein, the fluorine-containing monomer shown in formula (2) is preferably prepared according to the following method: a) methyl pyruvate and 2,4-pentanediol are reacted to obtain 2-methyl ester-2,4,6-trimethyl -1,3-dioxane; b) 2-methyl ester -2,4,6-trimethyl-1,3-dioxane is fluorinated and neutralized to obtain fluorine-containing carboxylic acid alkali metal salt; c) decomposing an alkali metal salt of a fluorine-containing carboxylic acid to obtain a fluorine-containing monomer repr...
Embodiment 1
[0069] Mix 102g of methyl pyruvate (1mol) and 104g of 2,4-pentanediol (1mol) in a 500mL three-necked flask, add 150ml of toluene and 5g of p-toluenesulfonic acid, reflux for 24 hours and evaporate the system through a water separator The water and the solvent were distilled under reduced pressure to obtain 35 g of the fluorinated precursor 2-methyl ester-2,4,6-trimethyl-1,3-dioxane. 5 grams of the obtained precursor were mixed in 200 ml of FC-75, 10% concentration of fluorine gas was passed through, and the reaction was carried out at 20° C. for 24 hours, and the reaction was tracked by GC-MS. After the reaction was completed, 5M aqueous sodium hydroxide solution was added to the reaction solution until the pH value was neutral, the aqueous phase was separated, concentrated and freeze-dried to obtain the sodium salt of perfluorocarboxylic acid. The obtained perfluorocarboxylic acid sodium salt was heated to 200°C in a nitrogen atmosphere to carry out a thermal decomposition re...
Embodiment 2
[0075] In a 100mL autoclave, 1.72g perfluoro(2-methylene-4,6-dimethyl-1,3-dioxane), 1.22g perfluoro(2-methylene-4-methyl- 1,3-dioxolane), perfluorodibutyryl peroxide (15mg) was dissolved in perfluorobenzene (10mL), cooled in acetone-dry ice bath, the system was evacuated and high-purity argon was circulated three times, 50 The reaction was heated at °C for 3 hours, and then heated at 60°C for 2 hours. After the system was cooled, the reaction solution was poured into acetone, and a colorless transparent solid precipitated out. The resin was washed three times with acetone, filtered, and vacuum-dried at 50° C. for 12 hours to obtain 2.83 g of white powder, which was an amorphous fluoropolymer.
[0076] Utilize nuclear magnetic resonance to detect the amorphous fluoropolymer obtained in embodiment 2, obtain 19 As a result of FNMR detection, the A / W ratio in the copolymer is 47 / 53.
[0077] Utilize differential scanning calorimetry (DSC) to detect the amorphous fluoropolymer ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com