Method for separating free fetal cells from peripheral blood of pregnant women
A technology for maternal peripheral blood and fetal cells, applied in chemical instruments and methods, analytical materials, material excitation analysis, etc., can solve the problems of small size, only identification, low enrichment rate of fetal cells, and reduce non-specific adsorption. , Improve the probability of acquisition, and improve the effect of capturing purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of fetal cell capture microfluidic chip
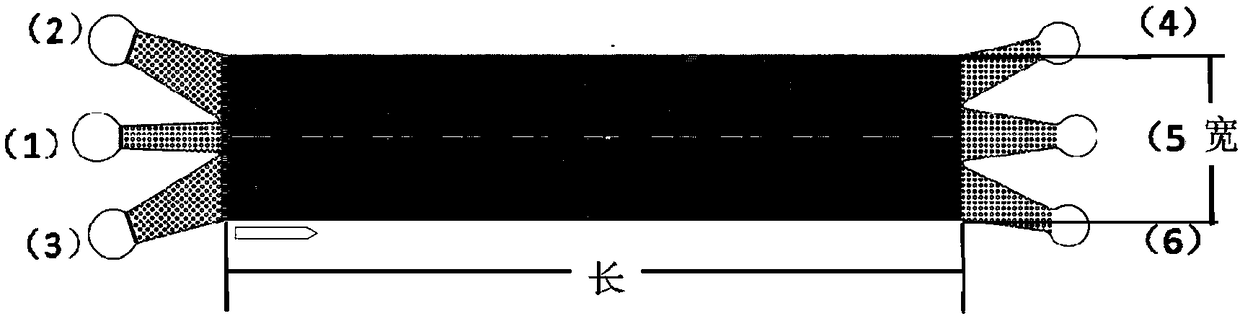
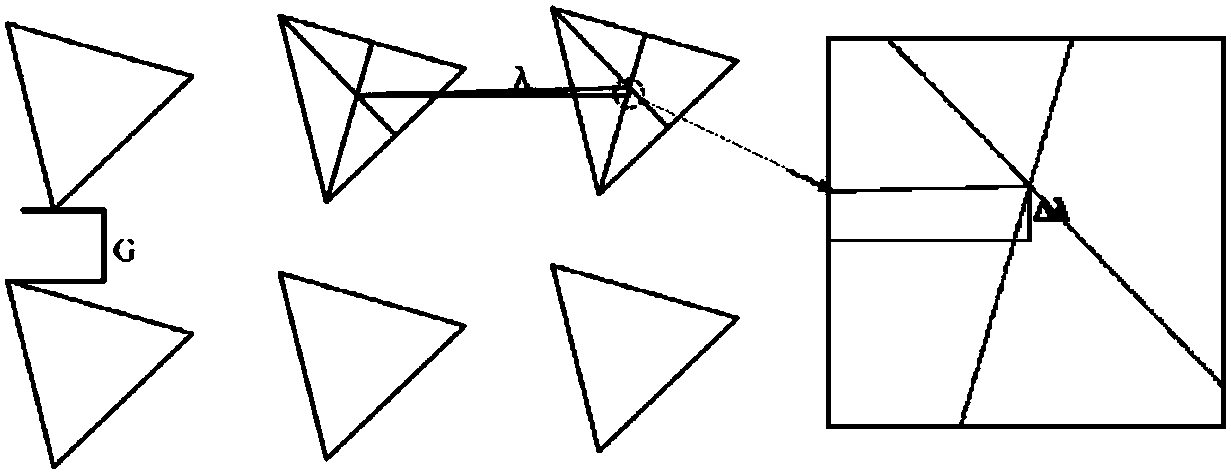
[0036] see figure 1, to make a microfluidic chip. The chip consists of two layers of PDMS and a layer of glass chip from bottom to top. The PDMS thick block, PDMS channel layer, and slide glass are sequentially used to form a complete chip by plasma bonding. The chip is equipped with three sample inlets (1), (2), (3) and three sample outlets (4), (5), (6), with a triangular microarray between the sample inlet and the sample outlet, The microarray arrangement adopts the DLD design principle, such as figure 1 shown.
[0037] In this embodiment, three sample inlets are located on the left side of the chip, and three sample outlets are located on the right side of the chip, and a 0.7mm punch pen is used to prepare the inlet and outlet ports.
[0038] In this embodiment, the chip size is designed to be 1 cm wide and 4.5 cm long.
[0039] In this embodiment, the horizontal distance G between the pillars is se...
Embodiment 2
[0041] Example 2 Simulated Fetal Cell Capture Characterization
[0042] The condition parameters and specific steps are as follows:
[0043] 1) Fluorescence pre-stained target cells (Ramos&K562) or control cells (WBCs) were resuspended in PBS buffer solution after cell counting;
[0044] 2) The sample is injected from the injection hole (1) through the injection system, and the buffer solution is injected through (2) and (3);
[0045] 3) Inject PBS buffer solution through the injection port (1, 2, 3) to clean the chip;
[0046] 4) The cells are counted and counted, and divided by the number of injected cells to calculate the capture efficiency;
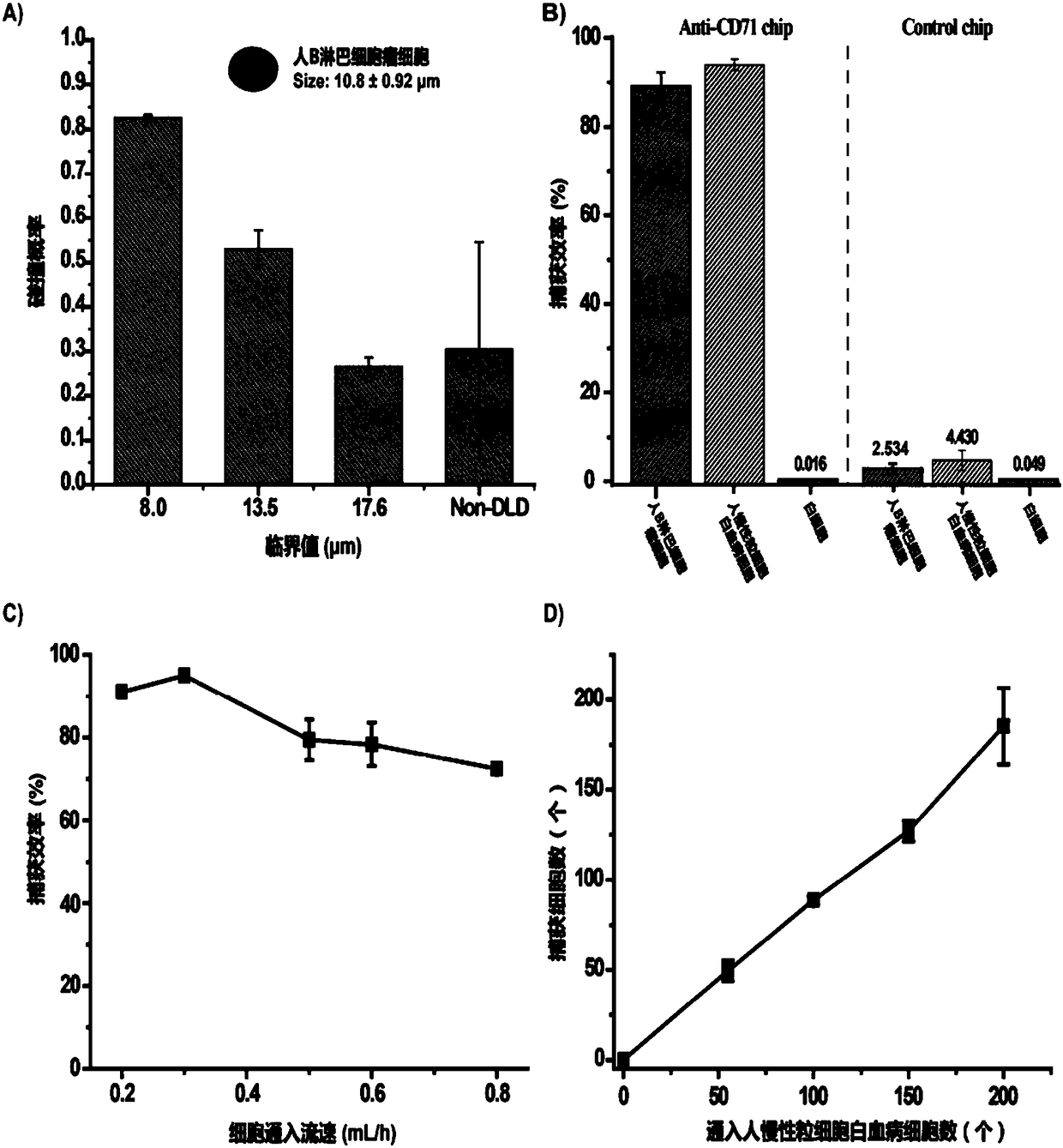
[0047] The transferrin CD-71 antibody is bound to the chip, and the efficiency of capturing target cells is greater than 80%, and the white blood cells are 0.016%.
[0048] In this example, the result is as image 3 shown.
[0049] image 3 Statistics for the capture efficiency of simulated fetal cells by the microfluidic chip. ...
Embodiment 3
[0051] Embodiment 3 contains the preparation of the mononuclear cell suspension of fetal cell
[0052] Two milliliters of peripheral blood from pregnant women was taken, diluted to 4 milliliters by adding PBS buffer, and the above sample was processed by percoll gradient centrifugation to obtain a mononuclear cell layer, washed, and resuspended to obtain a mononuclear cell suspension. The percoll density is 1.090, the centrifugal force is 400g, and the time is 30 minutes. Figure 5 Schematic diagram of gradient centrifugation of peripheral blood in pregnant women.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Critical diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com