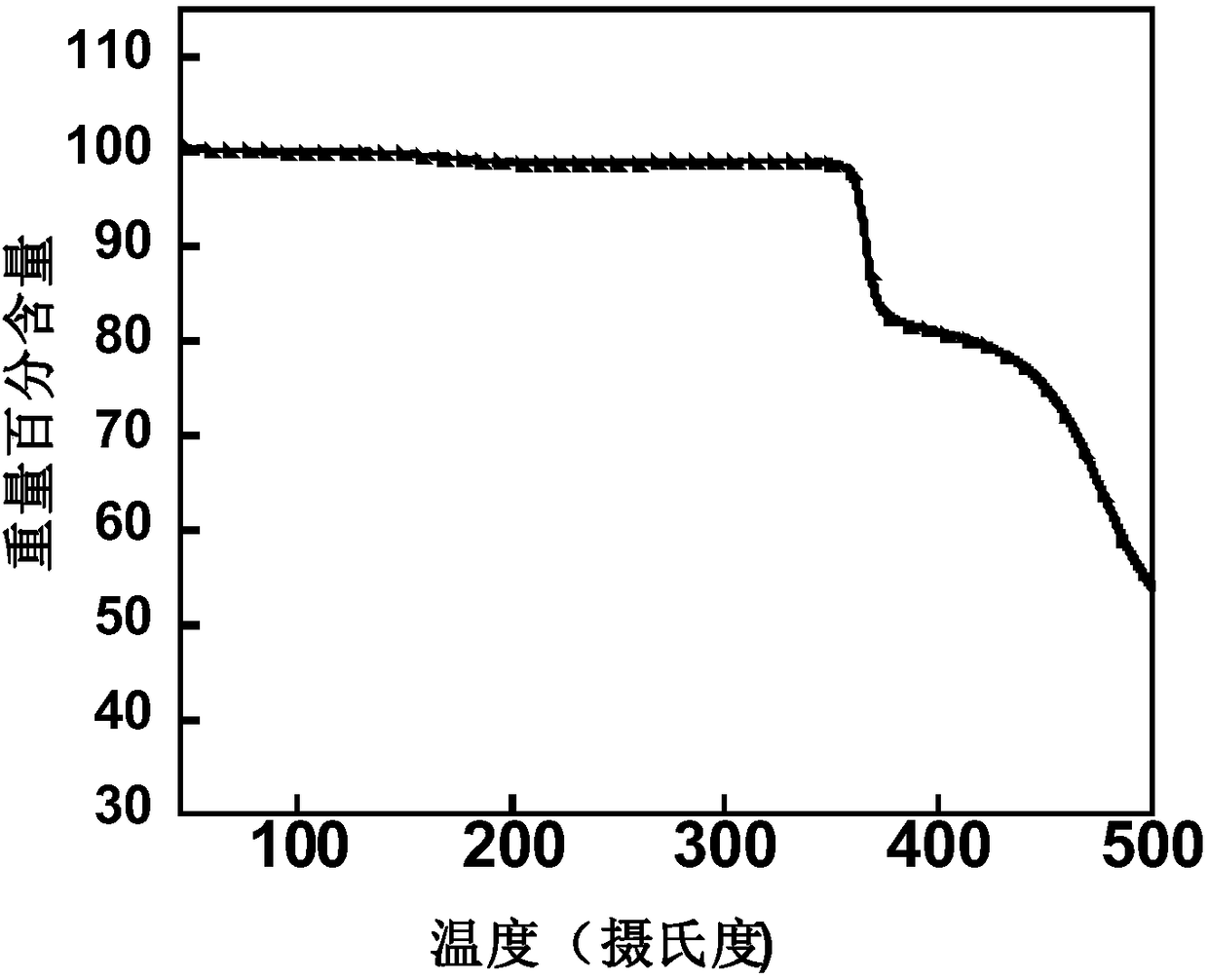
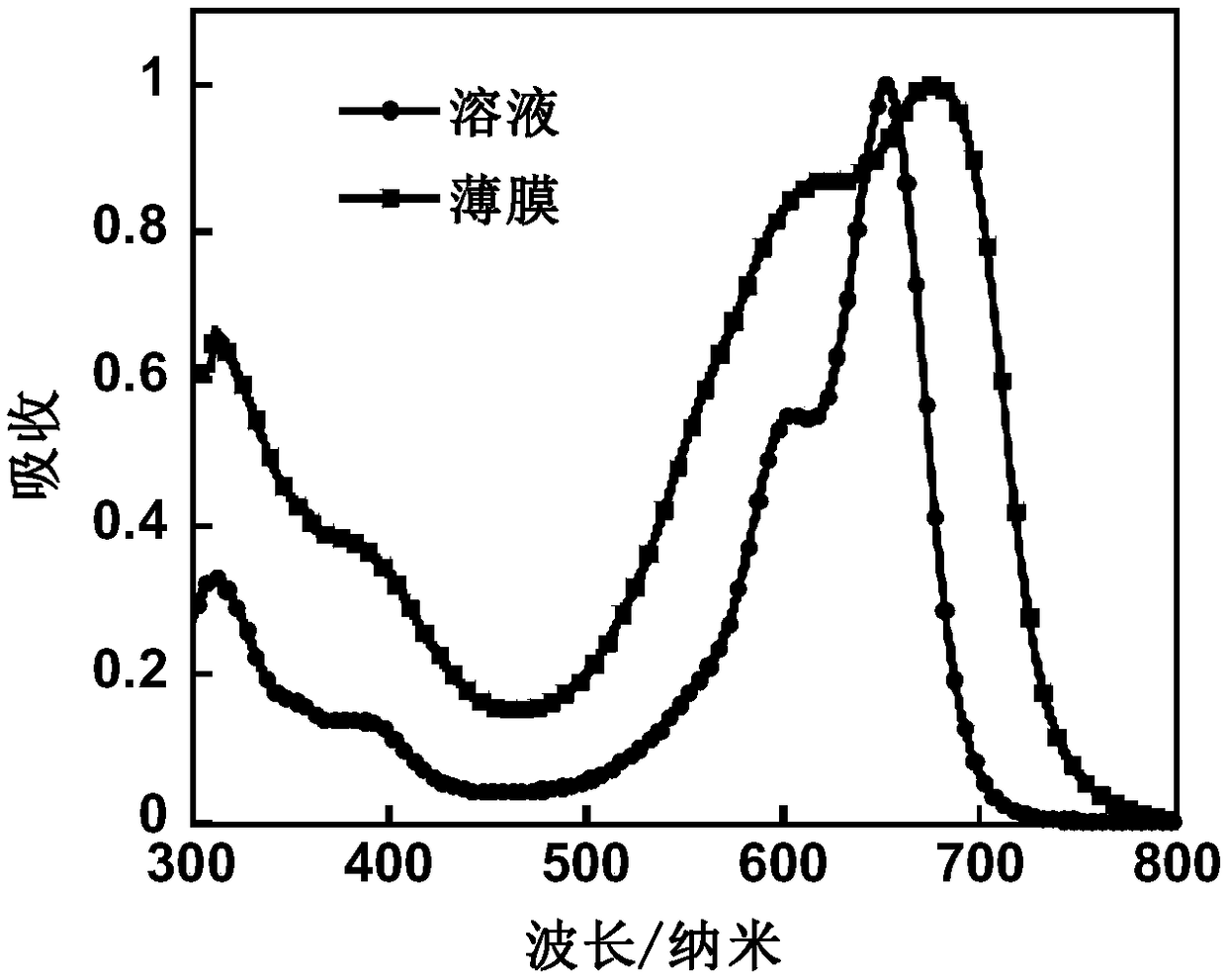
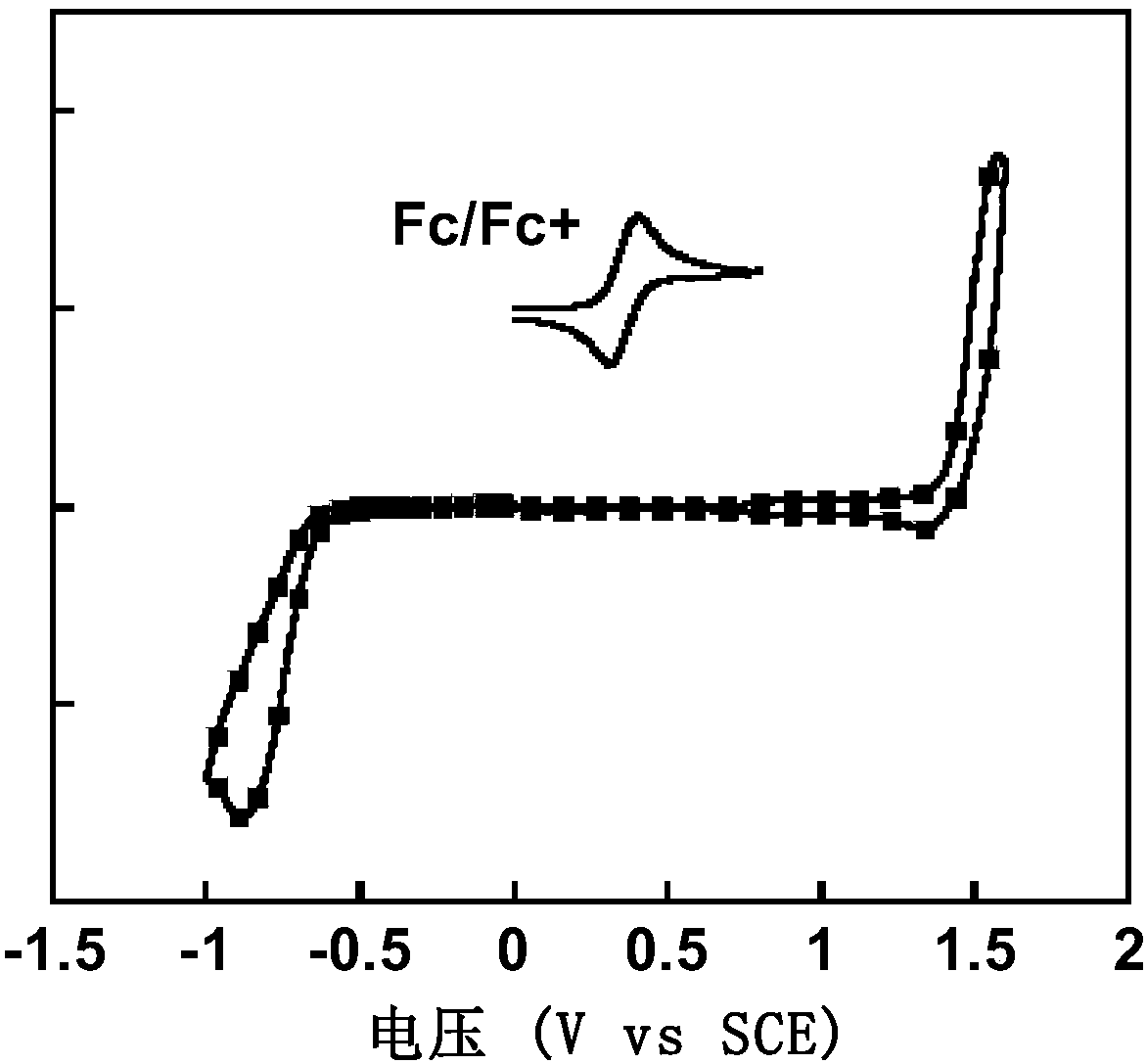
Organic conjugated small molecular material with terminal group containing naphthenic chain as well as preparation method and application thereof in solar cells
A technology containing cycloalkyl chains and cycloalkyl chains, applied in organic chemistry, circuits, photovoltaic power generation, etc., to achieve high photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of Compound 5,6,7,8-Tetralindone (2)
[0054] Prepare a 75mL dry pressure-proof bottle, pass argon into it, add compound (1) (3g, 14.9mmol), acetic anhydride (25mL) and triethylamine (15mL) into the pressure-proof bottle successively, under stirring, Ethyl acetoacetate (5.8 mL, 44.6 mmol) was added to the reaction solution, and the reaction was heated to 100° C. for 12 hours after aeration for 20 minutes. Afterwards, the reaction solution was cooled to room temperature, poured into ice water containing HCl (2N), a precipitate was formed, then HCl (5N) (30 mL) was added to the mixed solution, and the mixed solution was heated to reflux, reacted for 3 hours, Afterwards, the reaction was returned to room temperature, and the precipitated solid compound was filtered, washed with water, and dried, and then separated and purified by column chromatography, the eluent was dichloromethane, and the target compound (2) was obtained as a yellow solid. The yield was 66%. ...
Embodiment 2
[0057] Synthesis of Compound 5,6,7,8-tetrahydronaphthalenecyanindanone (HN)
[0058] Prepare a 100mL dry two-necked flask, pass into argon, add compound (2) (2g, 10mmol), malononitrile (2g, 30mmol) and ethanol (30mL) into the reaction flask successively, after stirring evenly, the Sodium acetate (1.1 g, 13 mmol) was added to the reaction solution and stirred at room temperature for 2 hours. Then pour the reaction solution into ice water, add concentrated HCl dropwise to the ice-water mixture, adjust the pH to 1-2, a large amount of precipitate is formed, then filter, wash with water, dry, and then use column chromatography to separate and purify, elute The solvent is dichloromethane, and the obtained product is recrystallized in ethanol to obtain a yellow needle product with a yield of 80%. 1 HNMR (500 MHz, CDCl3, δ): 8.30(s, 1H), 7.66(s, 1H), 3.66(s, 2H), 2.77(d, J=5.0Hz, 4H), 1.89-1.86(m, 4H ). 13 C NMR (125MHz, CDCl3, δ): 194.84, 166.62, 147.54, 147.41, 139.90, 138.20, 1...
Embodiment 3
[0061] Synthesis of Pentacondensed Ring Compound IDT-HN
[0062] Prepare a 100mL dry two-necked flask, pass through argon, add compound IDT-CHO (200mg, 0.21mmol), compound (HN) (155mg, 0.63mmol) and chloroform (30mL) into the reaction flask successively, at room temperature After stirring evenly, 1 mL of pyridine was added dropwise into the reaction liquid, and after aeration for 20 minutes, the reaction was heated to 65° C. for 12 hours. Post-reaction treatment: return the reaction to room temperature, and directly adopt column chromatography for separation and purification. The eluent is dichloromethane. The obtained compound is recrystallized in a mixture of chloroform and methanol to obtain the target compound IDT-HN. The yield 90%. 1 HNMR(500MHz, CDCl3,δ): 8.82(s,2H),8.35(s,2H),7.70(s,2H),7.69(s,2H),7.57(s,2H),7.15-7.11(dd, J=5.0Hz, J=5.0Hz, 16H), 2.92(d, J=5.0Hz, 8H), 2.58(t, J=7.5Hz, 8H), 1.84(s, 8H), 1.63-1.57(m, 8H),1.35-1.29(m,24H),0.87(t,J=7.5Hz,12H). 13 C NMR(1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com