Composite nano hemostatic material based on natural polysaccharide/short peptide and preparation method thereof
A hemostatic material and natural polysaccharide technology, applied in the field of nano-medical hemostatic materials, can solve the problems of limited hemostatic effect of carboxymethyl chitosan, long blood clot formation time, tens of seconds or even longer, etc. The effect of improving the happy life index, excellent hemostatic properties, and reducing the cost of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The target polypeptide was synthesized by Fmoc microwave-assisted solid-phase synthesis of polypeptide.
[0068] Step 1: redistill the DMF and piperidine used in the experiment.
[0069] Step 2: Use the software on the peptide synthesizer to calculate the amount of each amino acid, deprotecting agent, activating agent, activating base, and capping agent in the synthesized target polypeptide, weigh the corresponding medicines, and prepare a solution. To synthesize 0.25mM Ac-IIIQGK-NH 2 As an example, the amount of amino acid, resin, deprotecting agent, activator, activating base, and capping agent required is shown in the table below:
[0070] Table 3 The dosage and preparation method of each drug
[0071]
[0072]
[0073] After preparing the required solution according to the above table, put the swollen resin into a microwave reactor, use a deprotecting agent to remove the Fmoc group on the amino group, and then the exposed amino group and the carboxyl group o...
Embodiment 2
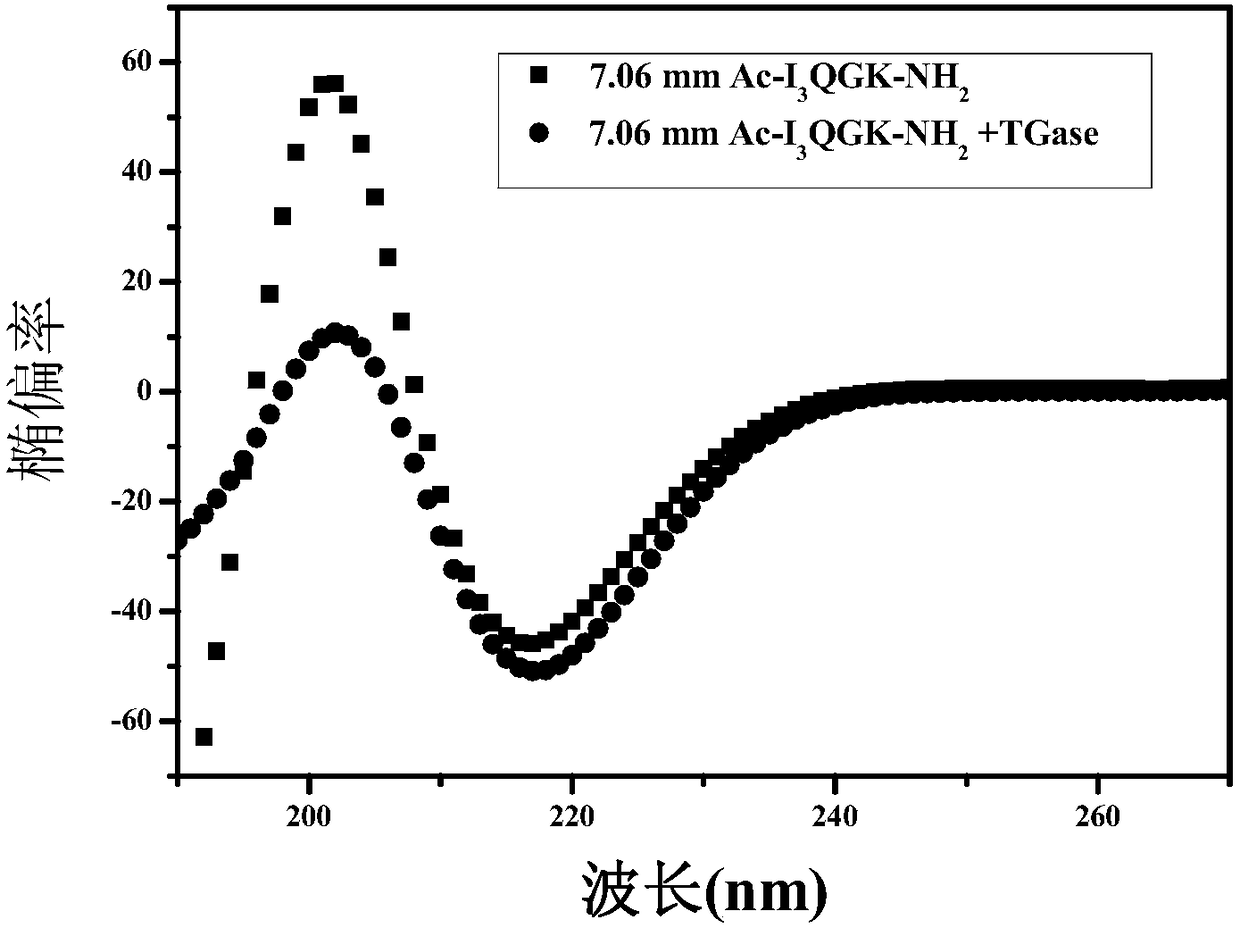
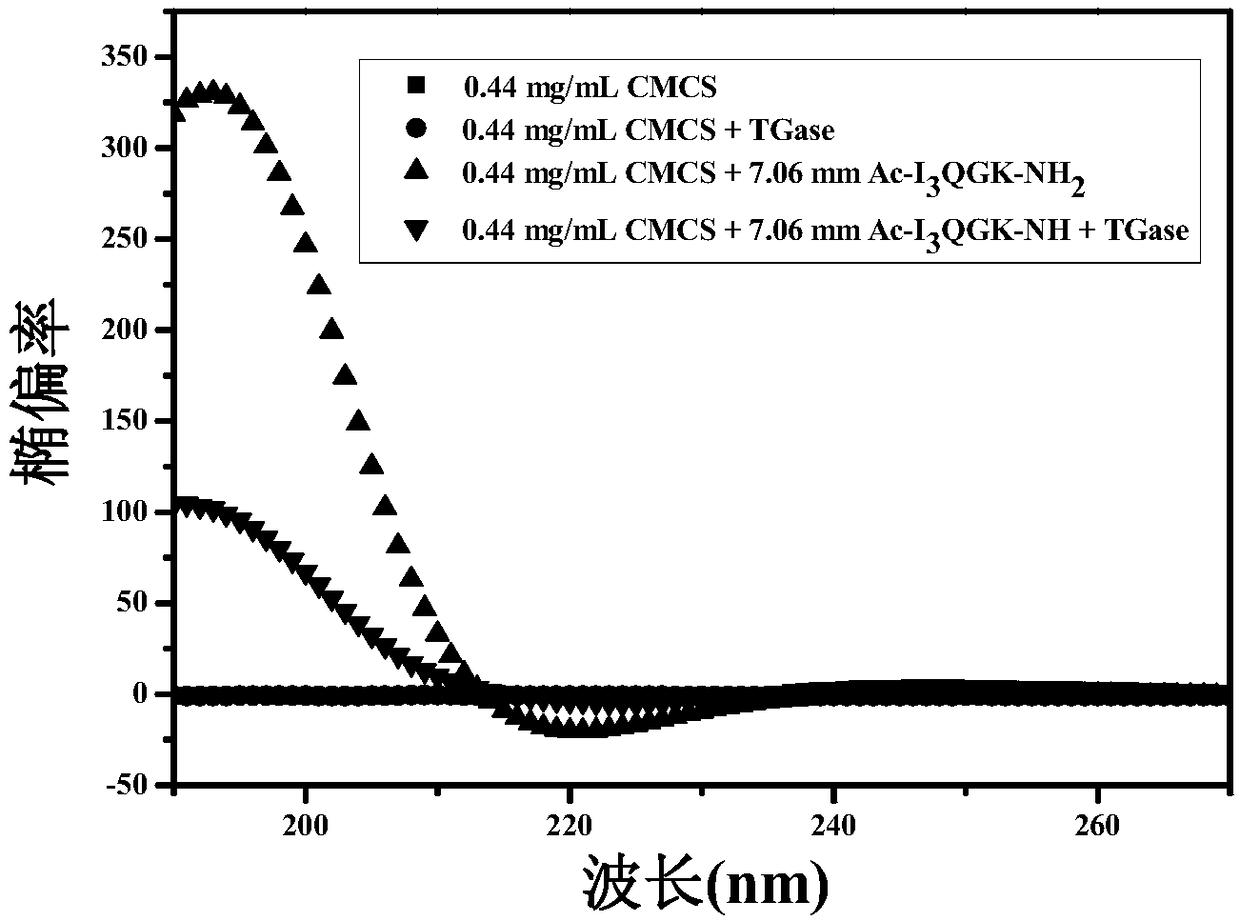
[0077] Example 2: Determination of the secondary structure of the natural polysaccharide / short peptide composite nanohemostatic material prepared in the present invention.
[0078] Preparation of 25mM Hepes solution: Weigh 2.9789mg Hepes salt, dissolve it in 400mL ultrapure water, then adjust its pH=7.4 with NaOH solution, then add ultrapure water to a 500mL volumetric flask to constant volume. Using Hepes solution as a solvent, weigh a certain amount of polypeptide to prepare a polypeptide solution, oscillate with a rotary oscillator for 5 minutes, sonicate for 30 minutes, heat in a water bath for 2 hours, and stand for 48 hours for use. Weigh a certain amount of CMCS and dissolve it in the Hepes buffer solution, add an equal volume of the CMCS solution to the polypeptide solution, so that the final concentrations of the polypeptide and CMCS are 7.06mM and 0.44mg / mL, respectively, and pour the above solution until the optical path length is 0.1 In the quartz cell of mm, the w...
Embodiment 3
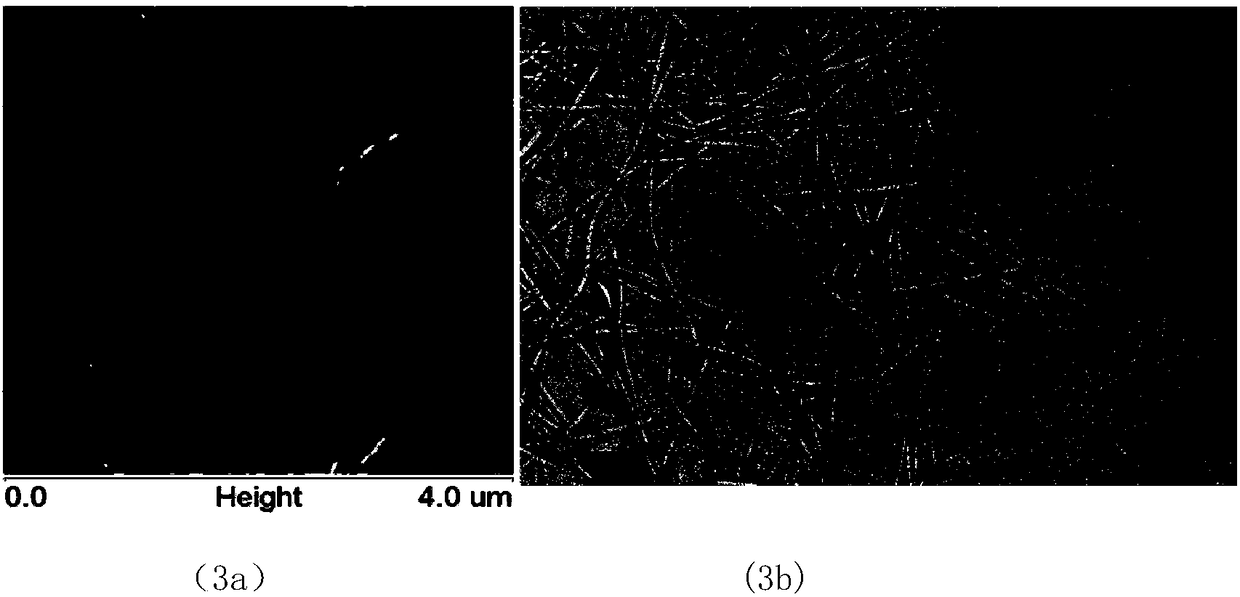
[0082] Ac-IIIQGK-NH in the present invention 2 And CMCS+Ac-IIIQGK-NH 2 The self-assembly morphology test, including atomic force microscope (AFM) and transmission electron microscope (TEM) test, the specific operation steps are as follows:
[0083] AFM test: Take a certain amount of the solution to be tested on the peeled mica sheet, absorb it for a certain period of time, then rinse it with a certain amount of ultrapure water, and then blow it with nitrogen until the mica sheet is dry. Put the obtained sample on the detection table, and use the tapping mode to scan the sample shape. The scanning angle is 0°, the scanning rate is 512×512, and the scanning rate is 1 Hz. The same sample needs to be scanned repeatedly to determine its true shape. From image 3 In (3a), (3b) and Figure 4 It can be seen from (4a) and (4b) that Ac-IIIQGK-NH 2 And CMCS+Ac-IIIQGK-NH 2 In Hepes buffer solution, it is a ribbon structure.
[0084] TEM test: use a pipette gun to draw a small amount...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com