Lithium-enriched manganese-based material precursor as well as preparation method, lithium-enriched manganese-based anode material and preparation method thereof, lithium battery
A lithium-rich manganese-based, positive electrode material technology, applied in battery electrodes, secondary batteries, chemical instruments and methods, etc., can solve problems such as difficult to remove, potential safety hazards of positive electrode materials, battery performance attenuation, etc., and achieve the effect of strong reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] ① Preparation of mixed salt solution: MnSO 4 ·H 2 O, NiSO 4 ·6H 2 O and CoSO 4 ·7H 2 O was dissolved in deionized water to prepare a mixed salt solution with a total ion concentration of 2 mol / L.
[0078] ② Precipitating agent preparation: prepare an aqueous solution containing 2mol / L sodium carbonate;
[0079] ③ Prepare complexing agent: prepare ammonia water as complexing agent, the concentration ratio of ammonia water and sodium carbonate is 0.05.
[0080]④ Carbonate co-precipitation reaction: Add the mixed salt solution, precipitant, and complexing agent prepared in steps ① to ③ into the continuous stirred tank reactor at the same time through the peristaltic pump, the feeding rate is 5mL / min, and the reaction temperature is 50°C. The pH was controlled at 8.0, the stirring speed was 500rpm / min, and after the feeding was completed, the reaction was continued at a reaction temperature of 50°C for 2h, aged for 10h, washed with deionized water for 5 times, suction...
Embodiment 2
[0085] The difference between this embodiment and embodiment 1 is only in step 5 without adding any additives, and the operation is as follows:
[0086] According to lithium and step ①, the total molar ratio of metal ions in the mixed salt solution is 1.25:0.8, weigh lithium carbonate, dry ball mill and mix evenly. Lithium carbonate is added in excess of 0.05wt% (that is, added weighed amount*1.05wt%) to make up for the loss of lithium during high-temperature sintering.
[0087] Put the homogeneously mixed precursor and lithium carbonate mixture in a sagger, place it in a muffle furnace, pass in dry air, raise the temperature to 450°C at a rate of 3.5°C / min, sinter for 4h, and continue to heat up to 900°C. Sintered for 15 hours, cooled naturally, crushed, dissociated, and sieved to obtain a lithium-rich manganese-like cathode material Li 1.2 mn 0.54 Ni 0.13 co 0.13 o 2 .
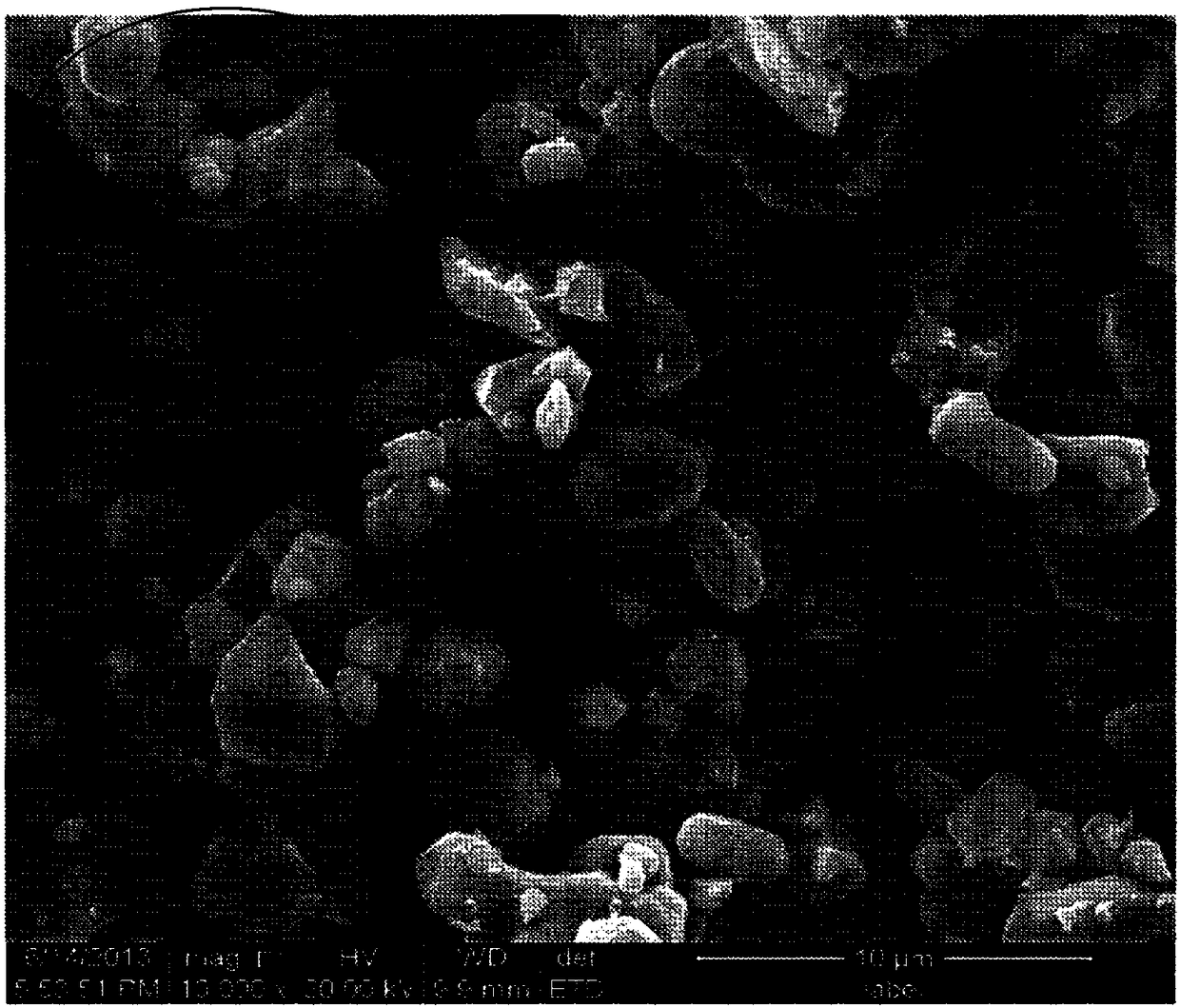
[0088] Observed by electron microscope, the grain shape can be seen in Figure 3b SEM image. Depe...
Embodiment 3
[0091] ① Preparation of mixed salt solution: MnSO 4 ·H 2 O, NiSO 4 ·6H 2 O and CoSO 4 ·7H 2 O was dissolved in deionized water to prepare a mixed salt solution with a total ion concentration of 3.5 mol / L.
[0092] ② Preparation of precipitant: preparation of an aqueous solution containing 3.5mol / L sodium carbonate;
[0093] ③ Prepare complexing agent: prepare ammonia water as complexing agent, the concentration ratio of ammonia water and sodium carbonate is 0.5.
[0094] ④ Carbonate co-precipitation reaction: Add the mixed salt solution, precipitant, and complexing agent prepared in steps ① to ③ into the continuous stirred tank reactor at the same time through the peristaltic pump, the feeding rate is 15mL / min, and the reaction temperature is 65°C. The pH is controlled at 8.5, the stirring speed is 1000rpm / min, after the feeding is completed, the reaction is continued at 65°C for 2h, aged for 5h, washed with deionized water for 5 times, suction filtered, dried in an oven...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com