Method for preparing epsilon-caprolactone by using carbon nanotube
A carbon nanotube and caprolactone technology, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of low efficiency and high cost of benzaldehyde, and achieve high catalytic activity and high industrial value. , the effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
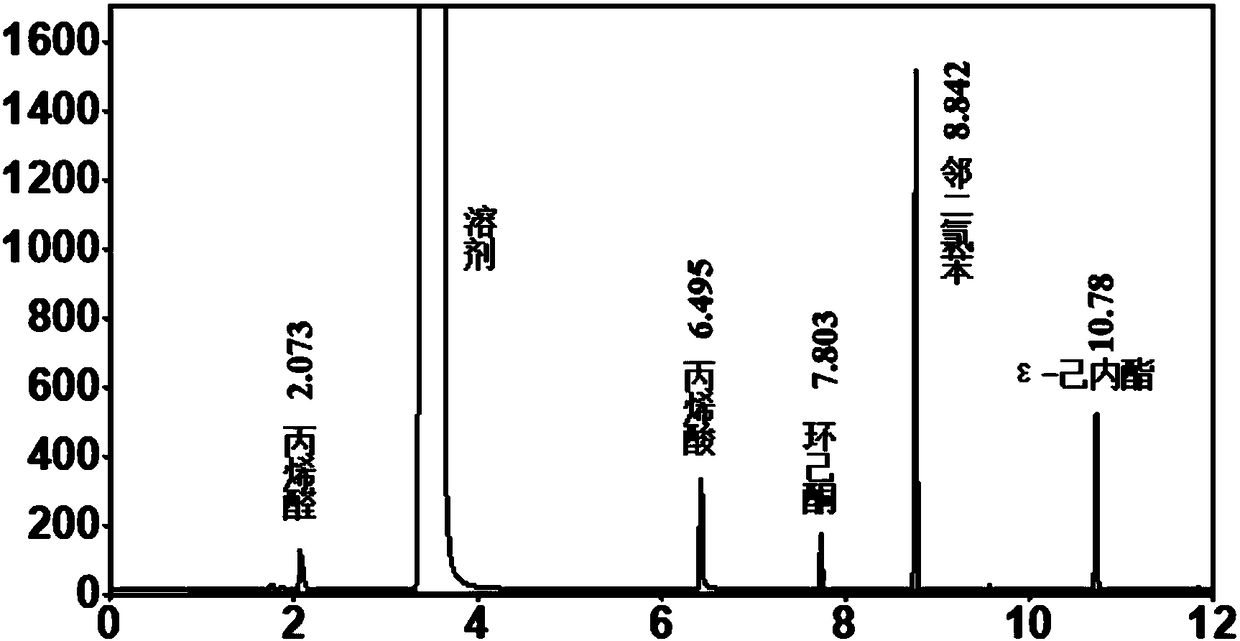
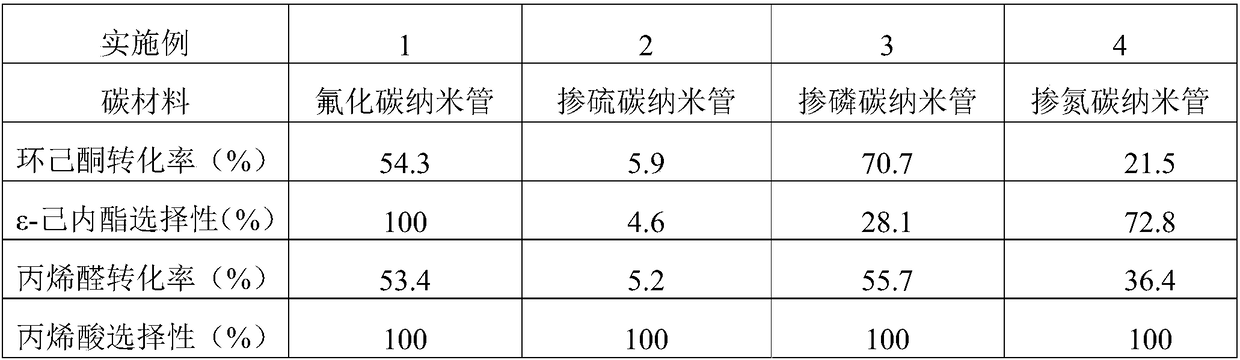
[0033] Add 17mL of 1,2-dichloroethane, 1.3g of o-dichlorobenzene (internal standard), 0.59g of cyclohexanone, 1.35g of acrolein and 50mg of the catalyst shown in Table 2 into the autoclave and stir Heat to 80°C, feed oxygen, start timing, and maintain its pressure at 1MPa during the reaction, stop timing after reacting for 4 hours, cool the reactor to room temperature, filter the liquid-solid phase mixture, and obtain a solid catalyst and containing unreacted A liquid mixture of reactants and reaction products. The liquid phase mixture is detected and analyzed with a gas chromatograph (GC) (see figure 1 ). The GC detection results are shown in Table 2 (the influence of different carbon materials on the Baeyer-Villiger oxidation reaction of cyclohexanone).
[0034] The sulfur-doped carbon nanotubes, phosphorus-doped carbon nanotubes, and nitrogen-doped carbon nanotubes used in this example are all self-made and made by chemical vapor deposition. The specific preparation metho...
Embodiment 4~7
[0043] Add 17mL of the solvent shown in Table 3, 1.3g o-dichlorobenzene (internal standard), 0.59g cyclohexanone, 1.35g acrolein and 50mg fluorinated carbon nanotubes (F content is 53.78at%) to Stir and heat the autoclave to 80°C, feed oxygen, start timing, and maintain the pressure at 1 MPa during the reaction, stop timing after reacting for 4 hours, cool the autoclave to room temperature, filter the liquid-solid phase mixture to obtain a solid Catalyst and liquid phase mixture containing unreacted reactants and reaction products. The liquid phase mixture was detected and analyzed by gas chromatography (GC). The GC detection results are shown in Table 3 (the influence of different solvents on the Baeyer-Villiger oxidation reaction of cyclohexanone).
[0044] table 3
[0045] Example
[0046] It can be seen from Table 3 that when 1,2-dichloroethane is used as the solvent, the selectivity of ε-caprolactone and acrylic acid and the efficiency of acrolein are the best...
Embodiment 8~11
[0048] Add 17mL 1,2-dichloroethane, 1.3g o-dichlorobenzene (internal standard), 0.59g cyclohexanone, 1.35g acrolein and 50mg fluorinated carbon nanotubes (F content is 53.78at%) Stir and heat to the high-pressure reactor to the temperature shown in Table 4, feed oxygen, start timing, and maintain the pressure at 1MPa during the reaction. After reacting for 4 hours, stop timing, cool the reactor to room temperature, and filter the liquid-solid phase mixture to obtain a solid catalyst and a liquid phase mixture containing unreacted reactants and reaction products. The liquid phase mixture was detected and analyzed by gas chromatography (GC). The gas chromatogram of the reaction solution after the reaction of embodiment 1 is as follows figure 1 shown. The GC detection results are shown in Table 4 (the influence of reaction temperature on the Baeyer-Villiger oxidation reaction of cyclohexanone).
[0049] Table 4
[0050] Example
[0051] As can be seen from Table 4, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com