Fluorine-fixing agent with high utilization rate and preparation method thereof
A technology of fluorine-fixing agent and utilization rate, applied in separation methods, chemical instruments and methods, gas treatment, etc., can solve the problem that the utilization rate of fluorine-fixing agent does not meet the industrial requirements, and achieve easy control of preparation process parameters and high reactivity , the effect of high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Get 8 grams of glucose, dissolve it in 45 milliliters of deionized water, make a solution, move to an autoclave equipped with a 100 milliliter polytetrafluoroethylene liner, place the autoclave in an oven, and let the autoclave stand. Raise to 180C with a heating rate of 10C / min, and react at a constant temperature for 6 hours. The precipitate was washed alternately with deionized water and ethanol. Dry at 80C for 12 hours to obtain carbon spheres.
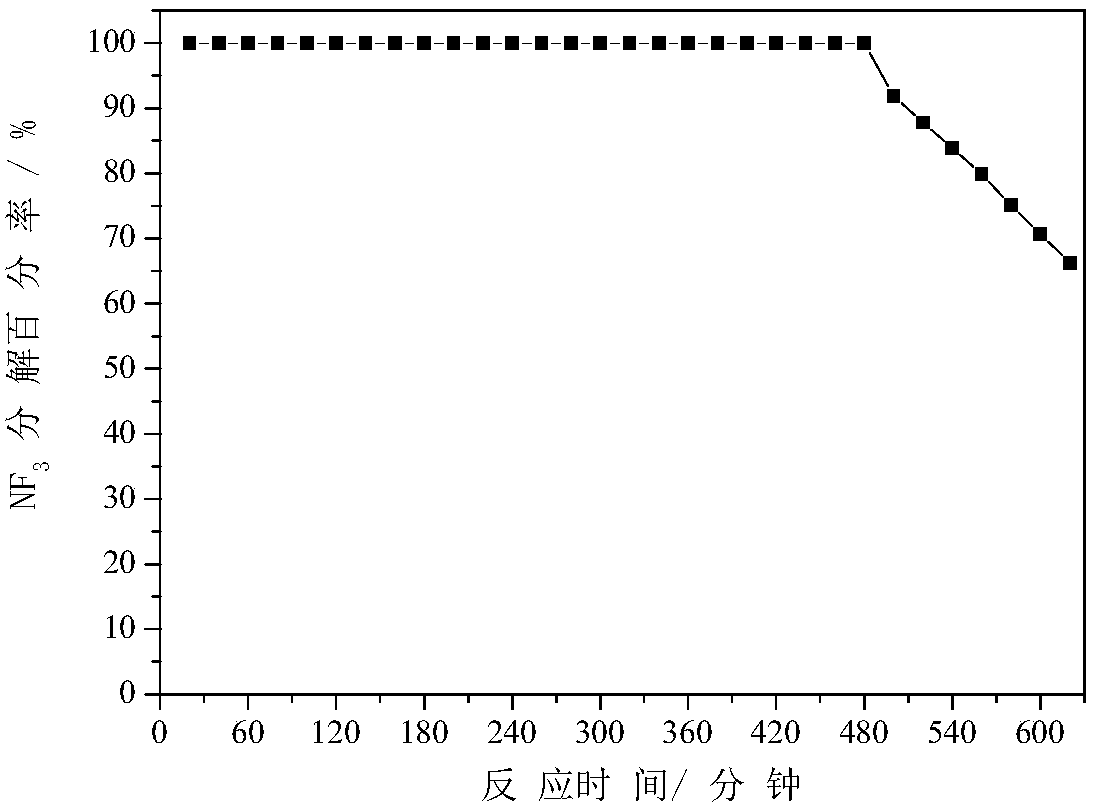
[0046] Take 2.5321 grams of aluminum nitrate and 1.6216 grams of urea, dissolve them in 45 milliliters of deionized water, add them to 1 gram of carbon spheres, stir and sonicate for 10 minutes, and transfer them to a self-pressurized autoclave equipped with a 100 milliliter polytetrafluoroethylene liner , put the autoclave in an oven, rotate the autoclave, raise the temperature to 120C at a rate of 10C / min, react at a constant temperature for 4 hours, wash the product with deionized water and ethanol, and dry at 80C for 1...
Embodiment 2
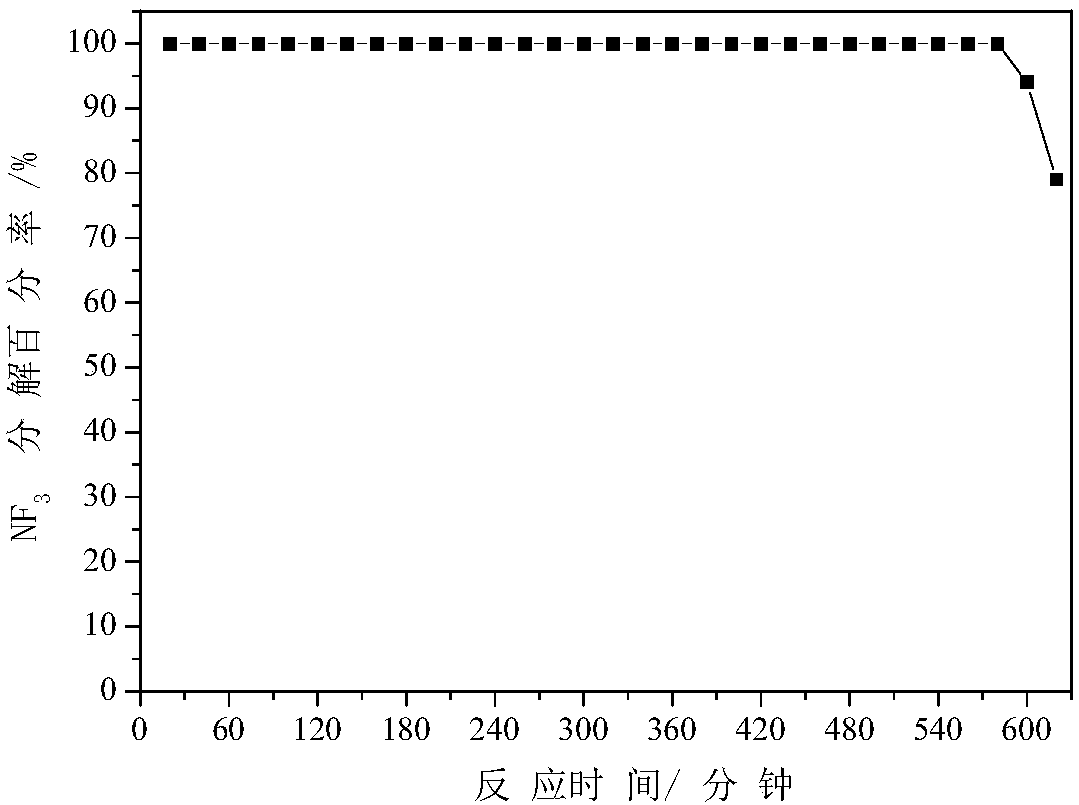
[0049] Get 0.1235 grams of cobalt nitrate and 0.1019 grams of urea, dissolve them in 45 milliliters of water, add them to 0.5 grams of the thin-shelled alumina prepared in "Example 1", stir and sonicate for 10 minutes, and move to a 100-ml In the autoclave with polytetrafluoroethylene liner, put the autoclave in the oven, rotate the autoclave, raise the temperature to 120C at a rate of 5C / min, react at constant temperature for 4 hours, and wash the product with deionized water at 80C Let dry for 12 hours. Raise the temperature to 600C at a rate of 5C / min in the air, and roast the above product for 4 hours at a constant temperature to obtain a thin-shelled Al as a defluorinating agent. 2 o 3 Load Co 3 o 4 , denoted as 5%Co / Al 2 o 3 (The mass of cobalt accounts for 5% of the mass of alumina). for NF 3 Anhydrous decomposition reaction, NF 3 Decomposition reaction condition is identical with embodiment one, NF 3 See the appendix for the decomposition percentage data fig...
Embodiment 3
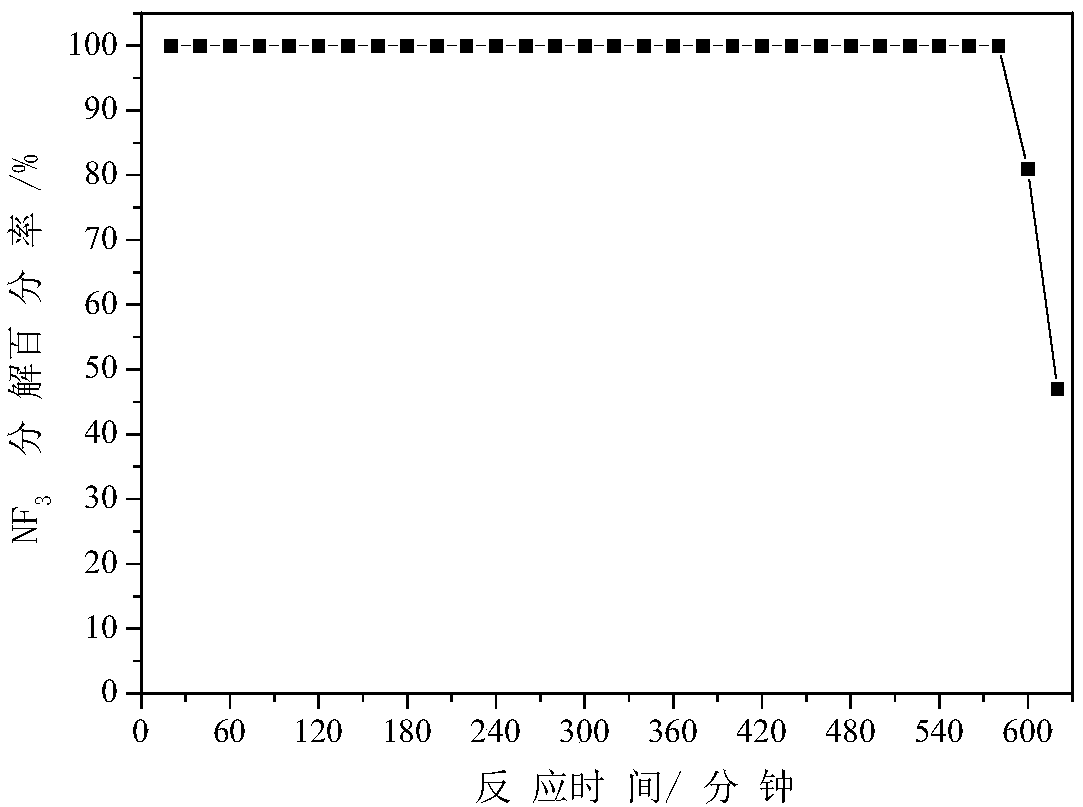
[0051] Get 0.1628 grams of manganese nitrate solution and 0.1093 grams of urea with a mass concentration of 50%, dissolve them in 45 milliliters of water, add them to 0.5 grams of the thin-shelled aluminum oxide prepared in "Example 1", stir and sonicate for 10 minutes , moved to an autoclave equipped with a 100 ml polytetrafluoroethylene liner, put the autoclave in an oven, rotate the autoclave, rise to 120C at a heating rate of 5C / min, and react at a constant temperature for 4 hours, the product Wash with deionized water and dry at 80C for 12 hours. In the air, the temperature was increased to 600C at a rate of 5C / min, and the above product was roasted at a constant temperature for 4 hours to obtain a thin-shell Al 2 o 3 Load Mn 2 o 3 , denoted as 5%Mn / Al 2 o 3 (The mass of manganese accounts for 5% of the mass of alumina). for NF 3 Anhydrous decomposition reaction, NF 3 Decomposition reaction condition is identical with embodiment one, NF 3 See the appendix for the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com