An anthraquinone-modified graphene quantum dot aag and its preparation method and its application in the preparation of lysine fluorescence detection reagent
A graphene quantum dot, fluorescence detection technology, applied in the directions of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of slow development, complex synthesis methods, low compound solubility, etc., and achieves convenient operation and synthesis methods. Simple, highly selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Synthesis of compound AAG
[0017] (1) Take 60 mL of 3.0 mg / mL graphene quantum dot aqueous solution in a 100 mL beaker, add dropwise 0.15 mL of N-hydroxysuccinimide and 1-(3-dimethylaminopropyl)-3 - A mixed solvent of ethylcarbodiimide hydrochloride was used as a catalyst, and it was activated by standing still for 10 min. Weigh 0.03 g of 1-aminoanthraquinone and dissolve it in 10 mL of alcohol solution, such as alcohol solution such as methanol or ethanol. Add dropwise to the above-mentioned activated graphene quantum dots, heat and ultrasonically disperse uniformly in a water bath at 40°C for 10 minutes, heat in a water bath at 55°C for 5 hours, and then stir at room temperature in the dark for 24 hours. Dialyze in 1000 mL deionized water for three days, and change the water every 3 hours to obtain anthraquinone-modified graphene quantum dots for the detection of lysine.
[0018] (2) Take 40 mL of 2.0 mg / mL graphene quantum dot aqueous solution in a 100 mL beaker, ...
Embodiment 2
[0019] Embodiment 2 (selective experiment)
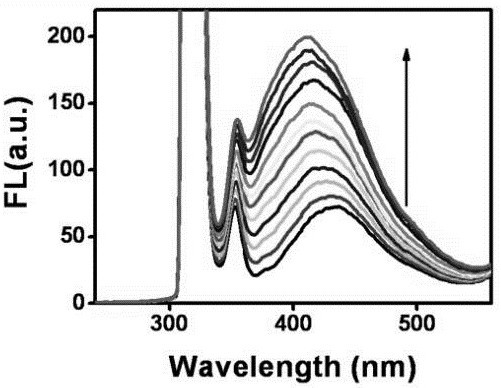
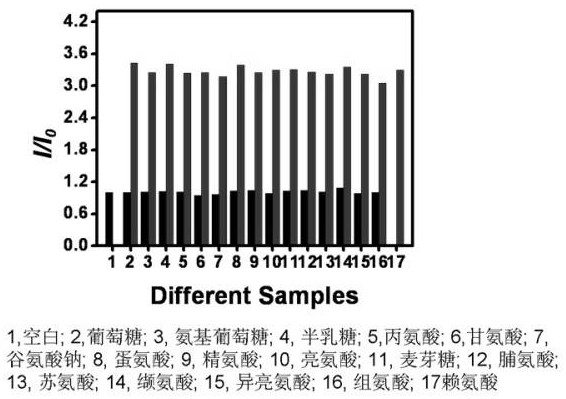
[0020] In the fluorescence experiment, the compound AAG was made into a 0.02 mg / mL aqueous stock solution, and the biomolecules were selected from lysine, alanine, arginine, glycine, glucose, glucosamine, lysine, maltose, lactose, sucrose, fructose and other substances , all experimental solutions were freshly prepared and tested immediately. Emitting at 432 nm, the biomolecules were tested separately. In the experiment, 2.5 mL of the stock solution was taken, and 1M biomolecules solution was added respectively. Test its fluorescence spectrum.
Embodiment 3
[0021] Example 3 Coexistence of Interfering Substances to Detect Lysine Experiment
[0022] In the fluorescence experiment, the compound AAG was prepared as a 0.025 mg / mL aqueous solution. Lysine was prepared as a 1M standard stock solution. Glycine, arginine, glucose, glucosamine, maltose, lactose, sucrose, fructose and other substances are selected as the biomolecules of the interfering substance. All experimental solutions were freshly prepared and tested immediately. In the interfering substance experiment, first add 5 times the interfering substance to the 0.025 mg / mL AAG aqueous solution to measure its fluorescence, then add 1M lysine to measure its fluorescence change. Fluorescence changes were detected at 432 nm.
[0023] Mechanism of the invention: due to the hydrogen bond interaction between lysine and the compound, the electron energy in the molecule changes and the fluorescence intensity changes, so as to achieve the purpose of detecting lysine. Glucose, glucos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com