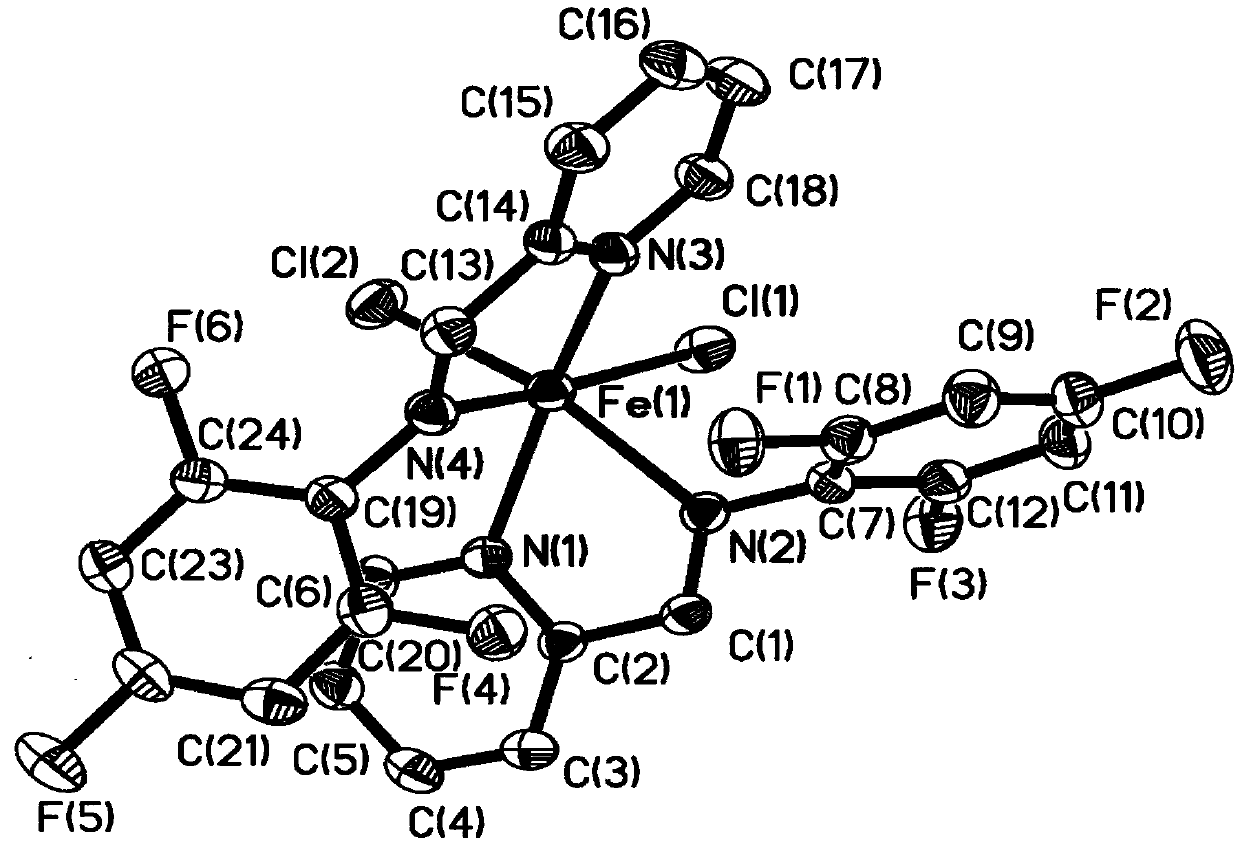
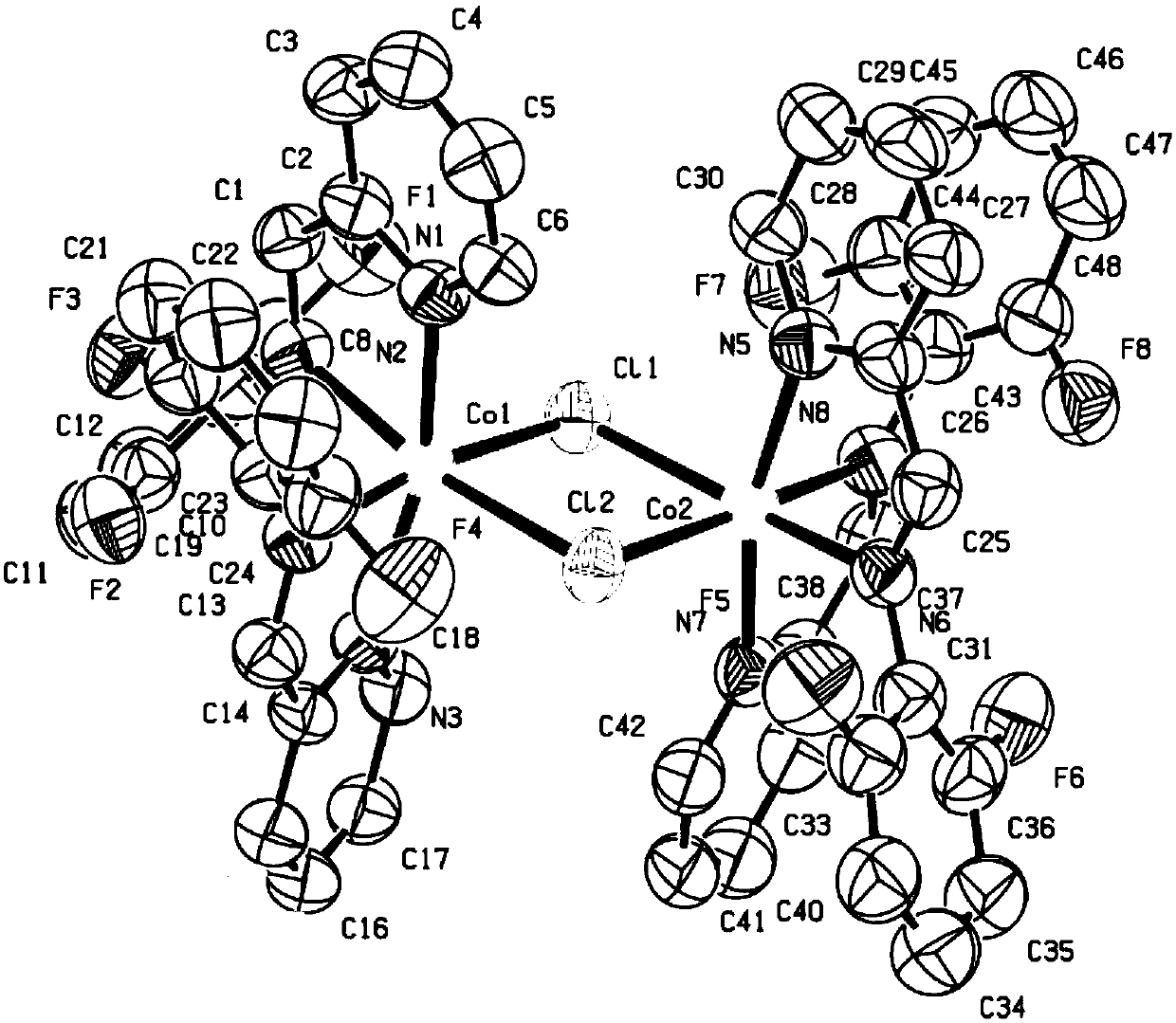
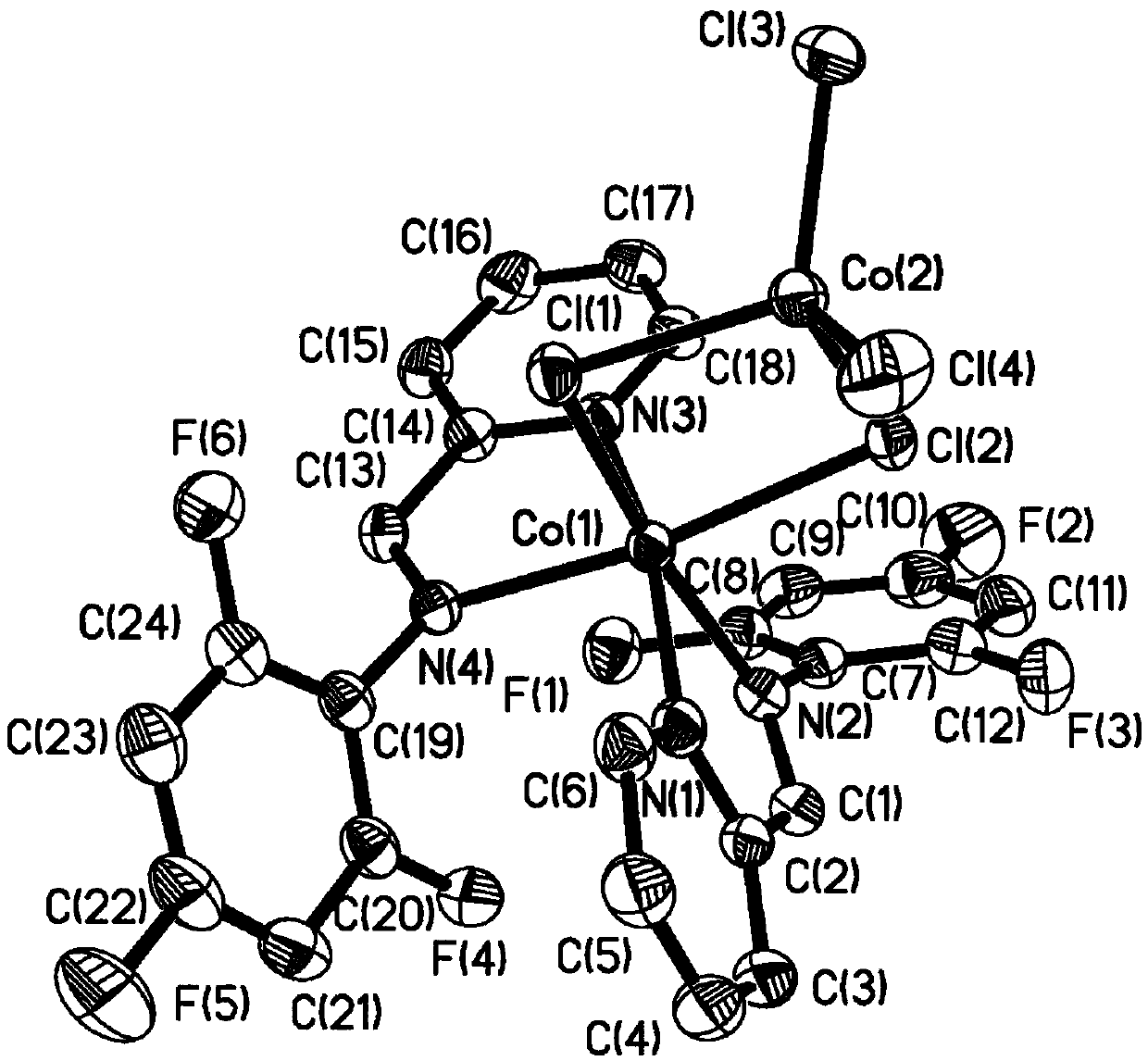
Fluorine-containing pyridine imine ligand, transition metal complex thereof and application of ligand to polyisoprene synthesis
A technology of polyisoprene and pyridinium, which is applied in the direction of compounds containing Group 8/9/10/18 elements of the periodic table, iron organic compounds, cobalt organic compounds, etc., can solve the problems of low activity, poor selectivity, Problems such as low molecular weight of synthetic polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 Preparation of fluorine-containing pyridine imine ligands
[0061] All ligands described here are obtained by condensation reactions of substituted aldehydes or ketones with primary amines. Complex amines need to be synthesized in advance. Many commercially available amines and substituted aldehydes and ketones can be subjected to one-step synthesis of ligands (imine condensation).
[0062] (1) Synthesis of pyridine imine ligand compound L1
[0063]
[0064]Add 2-difluoroaniline (1.03g, 9.24mmol, 1.00eq.) to a 25mL two-necked flask, replace it with Ar three times, then add pyridine-2-carboxaldehyde (1.00g, 9.34mmol, 1.01eq.) Dichloromethane solution 10mL, formic acid (0.1mL), reflux reaction at 50°C, TLC tracking detection until 2-difluoroaniline raw material disappeared. The solvent was spin-dried, and recrystallized five times with 40 mL of n-hexane to obtain 1.21 g of a white solid with a yield of 64%.
[0065] (2) Synthesis of pyridine imine liga...
Embodiment 2
[0077] The synthesis of embodiment 2 fluorine-containing pyridinium transition metal complexes
[0078] (1) Synthesis of pyridinium iron complex 1a
[0079]
[0080] In the glove box, add anhydrous FeCl to a 10 mL Schlenk tube 2 (95.0mg, 0.75mmol, 1eq.), the pyridine imine ligand compound L1 (150.0mg, 0.75mmol, 1eq.) prepared in Example 1, and then added 5mL of anhydrous DCM, and stirred at room temperature for 2 days. After the reaction is over, dissolve the precipitated solids with 10mL×2 anhydrous DCM and filter them separately to obtain a purple-black liquid, drain the remaining DCM; then wash twice with 20mL of anhydrous n-hexane, remove the supernatant and The residue was dried to give a purple solid in 71% yield.
[0081] (2) Synthesis of pyridinium iron complex 2a
[0082]
[0083] In the glove box, add anhydrous FeCl to a 10 mL Schlenk tube 2 (95.0mg, 0.75mmol, 1eq.), the pyridine imine ligand compound L2 (150.0mg, 0.75mmol, 1eq.) prepared in Example 1, and ...
Embodiment 3
[0111] Take a 25mL Schlenk bottle, add cocatalyst MAO (4mmol, 500eq.), toluene 5mL successively therein, complex 1a (8μmol, 1eq.) is dissolved in 1mL dichloromethane, this mixture is stirred at room temperature for 2min, then Isoprene monomer (20mmol, 2500eq.) was added, and after polymerization at 25°C for 2h, 1M methanolic hydrochloric acid solution was added to terminate the reaction. Pour the viscous polymer solution into 50 mL of ethanol and allow the polymer to settle out. The polymer after the supernatant was discarded was washed with distilled water and ethanol in turn, and the obtained polymer was dried in a vacuum oven at 40° C. to a constant weight to obtain the product with a yield of 78%. The reaction selectivity was 59% cis-1,4 and 41% 3,4-polyisoprene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com