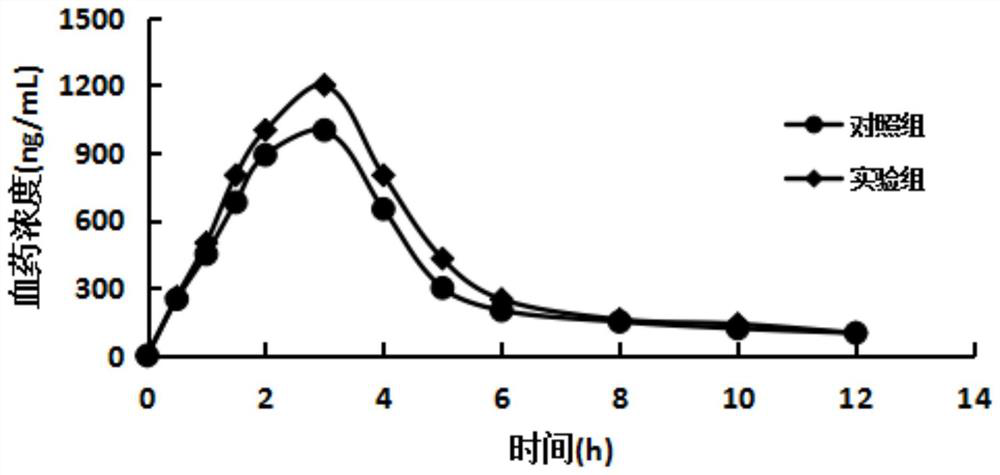
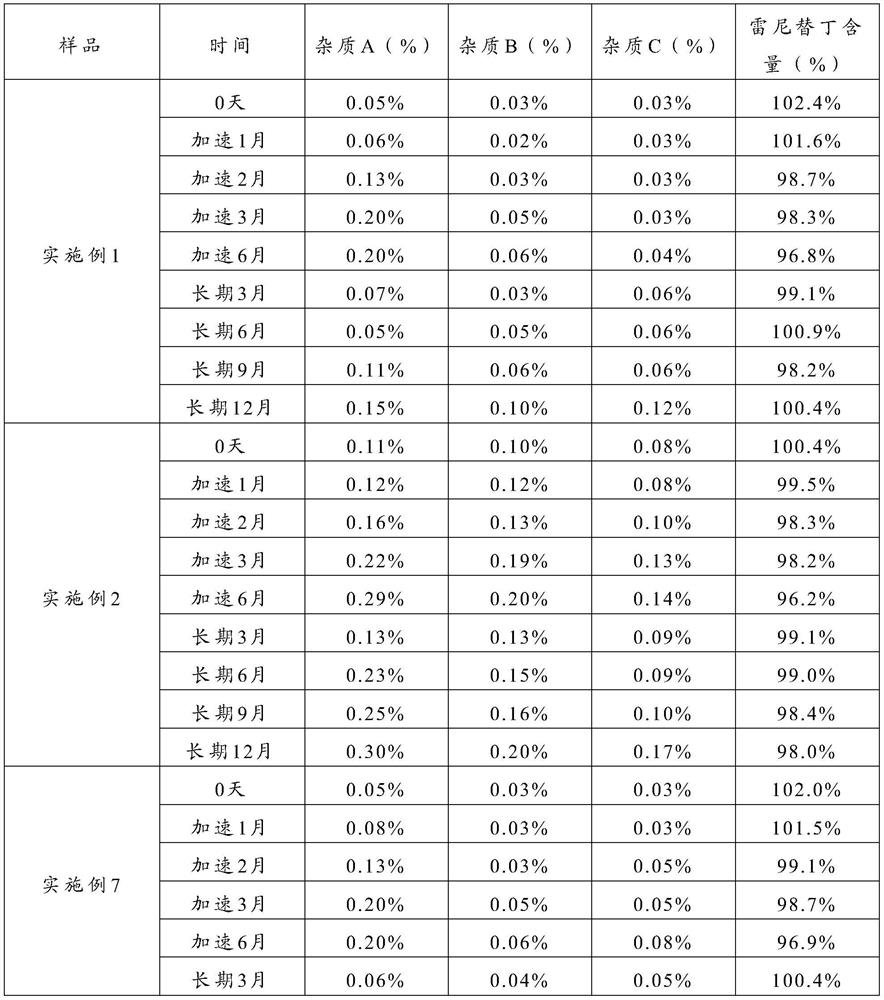
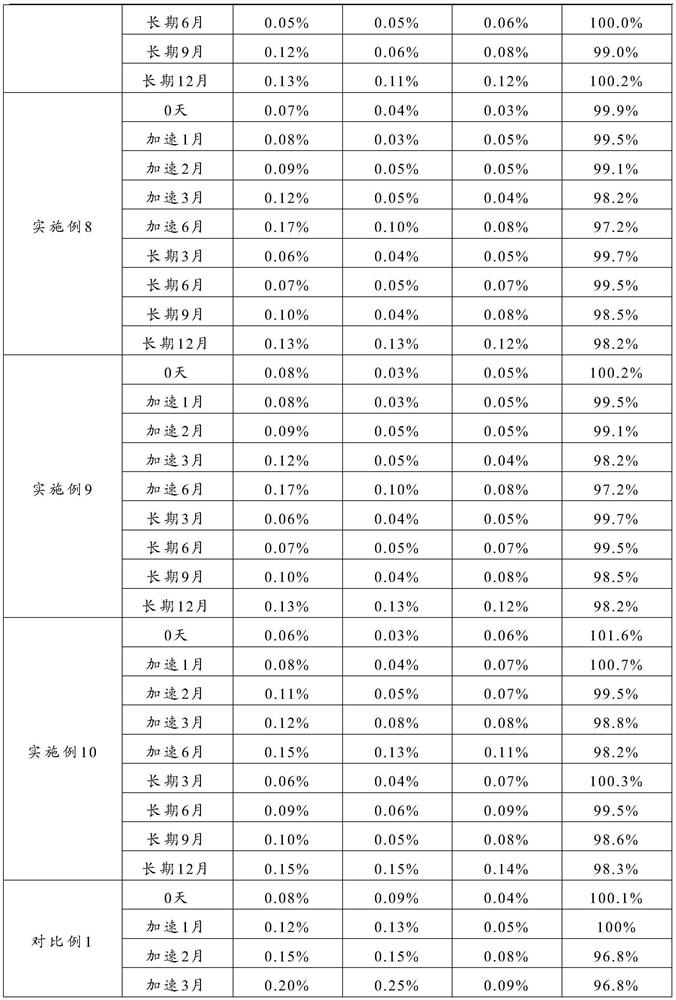
The New Indication of Oral Ranitidine in the Treatment of Erosive Esophagitis
A technology for ranitidine and esophagitis, applied in the field of medicine, can solve the problems of no effective treatment for erosive esophagitis, reduce vitamin B12 absorption, affect the health of patients, etc., achieve long drug action time and high bioavailability , to avoid the effect of indigestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] In a second aspect, the present invention provides a method for preparing ranitidine, the method comprising the following steps:
[0045] Step 1, 2-[[[5-(dimethylamino) methyl-2-furan] methyl] thio] ethylamine and N-methyl-1-methylthio-2-nitroethyleneamine respectively Dissolved in a solvent to make a solution, mix the two, and heat to a certain temperature to react.
[0046] According to one and a preferred embodiment of the present invention, the solvent is an organic solvent and / or water.
[0047] In a further preferred embodiment, the solvent is water, preferably distilled water.
[0048] In the present invention, impurity B For the unreacted 2-[[[5-(dimethylamino)methyl-2-furan]methyl]sulfanyl]ethylamine, the inventor found through research that distilled water is selected as the solvent for dissolving raw materials, which is beneficial to The reaction time is shortened, the yield is increased, and the synthesis reaction is fully carried out, so the content of ...
Embodiment approach
[0049] According to a preferred embodiment of the present invention, the 2-[[[5-(dimethylamino)methyl-2-furan]methyl]sulfanyl]ethylamine solution and N-methyl-1-methylthio The molar ratio of the base-2-nitrovinylamine solution is (1.0-3.0):1, preferably (1.1-2.5):1, more preferably (1.2-2.0):1.
[0050] In the present invention, impurity C For unreacted N-methyl-1-methylthio-2-nitroethyleneamine, the inventor found through research that 2-[[[5-(dimethylamino)methyl-2-furan] Methyl]thio]ethylamine and N-methyl-1-methylthio-2-nitroethyleneamine are set to (1.0~3.0):1, preferably (1.1~2.5):1, more preferably (1.2~2.0): The molar ratio of 1 can make the raw material react fully, the surplus is little, effectively reduces the content of impurity B and impurity C, thereby improves the medicine stability of the prepared ranitidine.
[0051] In a further preferred embodiment, the 2-[[[5-(dimethylamino)methyl-2-furan]methyl]thio]ethanamine solution and N-methyl-1-methylthio- 2-Nitro...
Embodiment 1
[0240] The preparation of embodiment 1 ranitidine
[0241] (1) 170g of 2-[[[5-(dimethylamino)methyl-2-furan]methyl]sulfanyl]ethylamine and 90.3g N-methyl-1-methylthio-2-nitro Vinylamine was dissolved in 100ml of distilled water respectively to make a solution. Take the same volume (50mL respectively) of the configured solution, and mix it dropwise at a rate of 2.4mL / min and a stirring rate of 180r / min. Afterwards, the temperature was raised to 42° C. at a heating rate of 2.5° C. / min, and kept for 6 hours until the reaction was completed, and an orange-yellow reaction liquid was obtained;
[0242] (2) Add 50mL, 2mol / L hydrochloric acid to the above reaction solution, then adjust the pH to 3.5, then add 200mL methylene chloride to the reaction solution for extraction, mix well, let stand, and separate to obtain an organic phase and an aqueous phase , wherein the water phase is 180mL; adopt 15% potassium carbonate solution to adjust the pH of the water phase to 9, then add 720...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com