Terbiphenyl bisphosphine tricoordinate cuprous halide complex and its synthesis method and application
A technology of terphenylbisphosphine and cuprous halide, applied in the field of terphenylbisphosphine tricoordinated cuprous halide complex and its synthesis, achieving the effects of high yield, easy operation and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
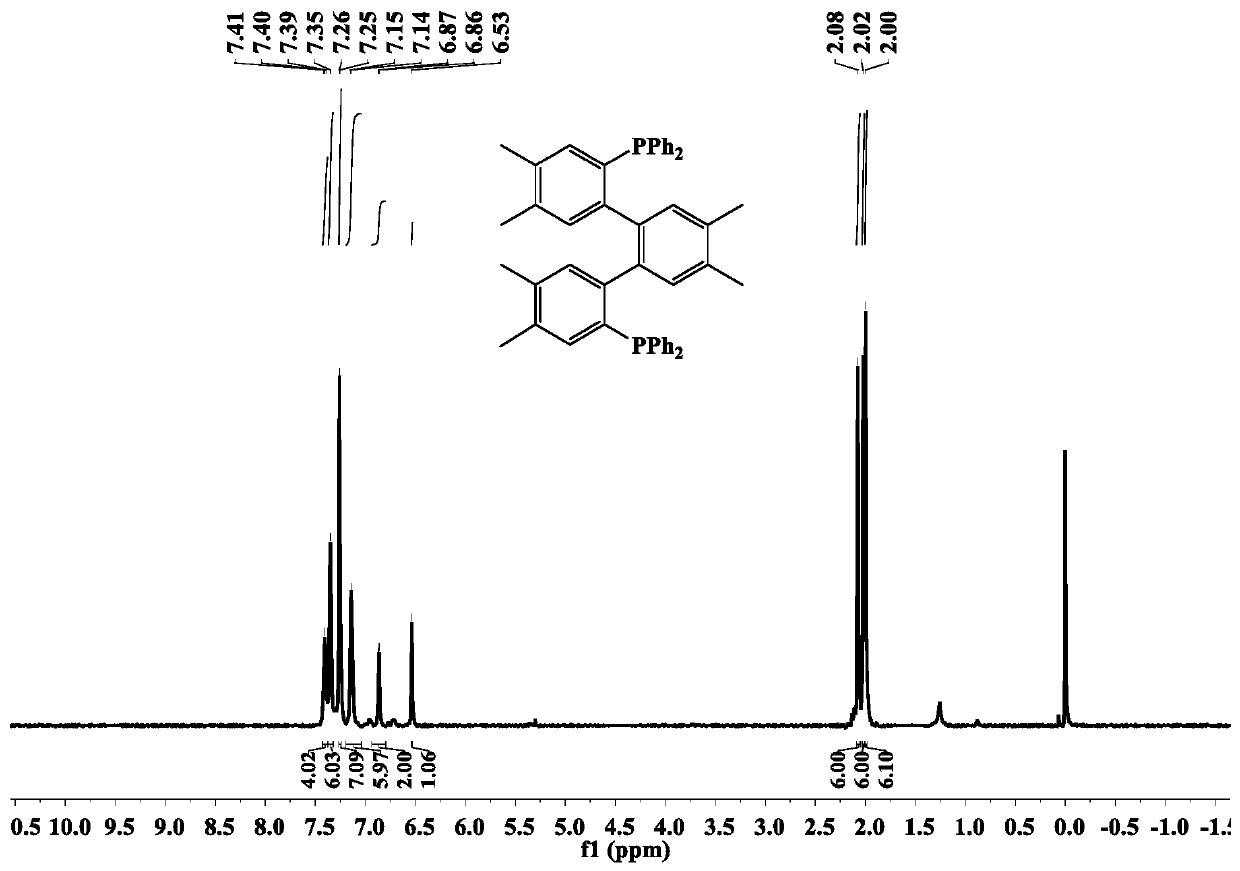
[0041] The 4,4',4",5,5',5"-hexamethyl-(1,1':2',1"-terphenyl)-2,2"-bis(diphenyl The synthetic method of base phosphine) ligand (dbdp), comprises the steps:
[0042] A three-necked flask containing 1,2-dimethyl-4,5-dibromobenzene (1 g, 3.81 mmol) was added 20 mL THF under vacuum, the temperature was lowered to -78 °C, and 2.02 mL normal Butyllithium n-hexane solution (2.5mol / L, 5.06mmol), keep the temperature at -78°C, stir for 1h, add diphenylphosphine chloride (0.56g, 2.53mmol), continue stirring for 2h, then warm to room temperature , after stirring for 12 hours, add 15 mL of water to quench the reaction, extract with dichloromethane to obtain the organic phase, dry and evaporate the organic solvent to obtain a yellow oily liquid, and then use petroleum ether:dichloromethane=4:1 (volume ratio) as the developer 0.55 g of a white solid product was obtained by column chromatography separation, and the yield was 30.5%.
[0043] IR(KBr,ν / cm -1 ) test results are as follows: 307...
Embodiment 2
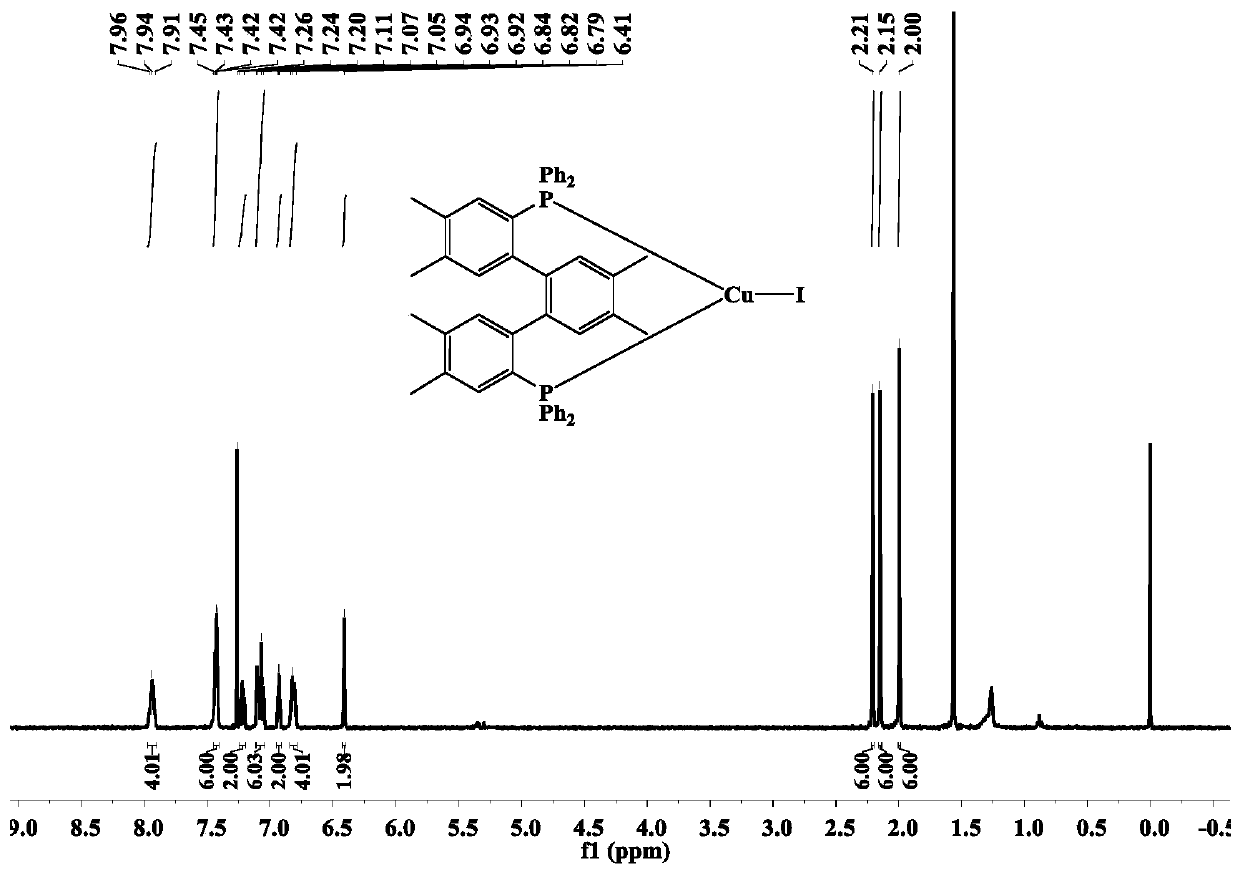
[0047] The terphenyl bisphosphine tricoordinate cuprous iodide complex (complex 1) of the present embodiment is prepared by the following method, including the following steps:
[0048] Add dbdp (143mg, 0.21mmol) and cuprous iodide (0.21mmol) synthesized in Example 1 into 20mL of dichloromethane, react for 5h, filter, suspend and dry the solvent under reduced pressure to obtain a light yellow-green solid. Then the light yellow-green solid was dissolved in a mixed solvent of 10 mL of dichloromethane / ethanol (V:V=3:1), and a colorless crystal was finally obtained by solvent evaporation at room temperature, that is, the complex 1. The yield 80%.
[0049] The relevant test data of the complex 1 synthesized above are as follows:
[0050] 1 H NMR (600MHz, CDCl 3 ) test results are as follows: δ: 7.96~7.91(m,4H), 7.45~7.42(m, 6H), 7.24~7.20(m,2H), 7.11~7.05(m,6H), 6.94~6.92(m,2H ),6.84~6.79(m,4H), 6.41(s,2H),2.21(s,6H),2.15(s,6H),2.00(s,6H).
[0051] 31 P NMR (240M, CDCl 3 ) t...
Embodiment 3
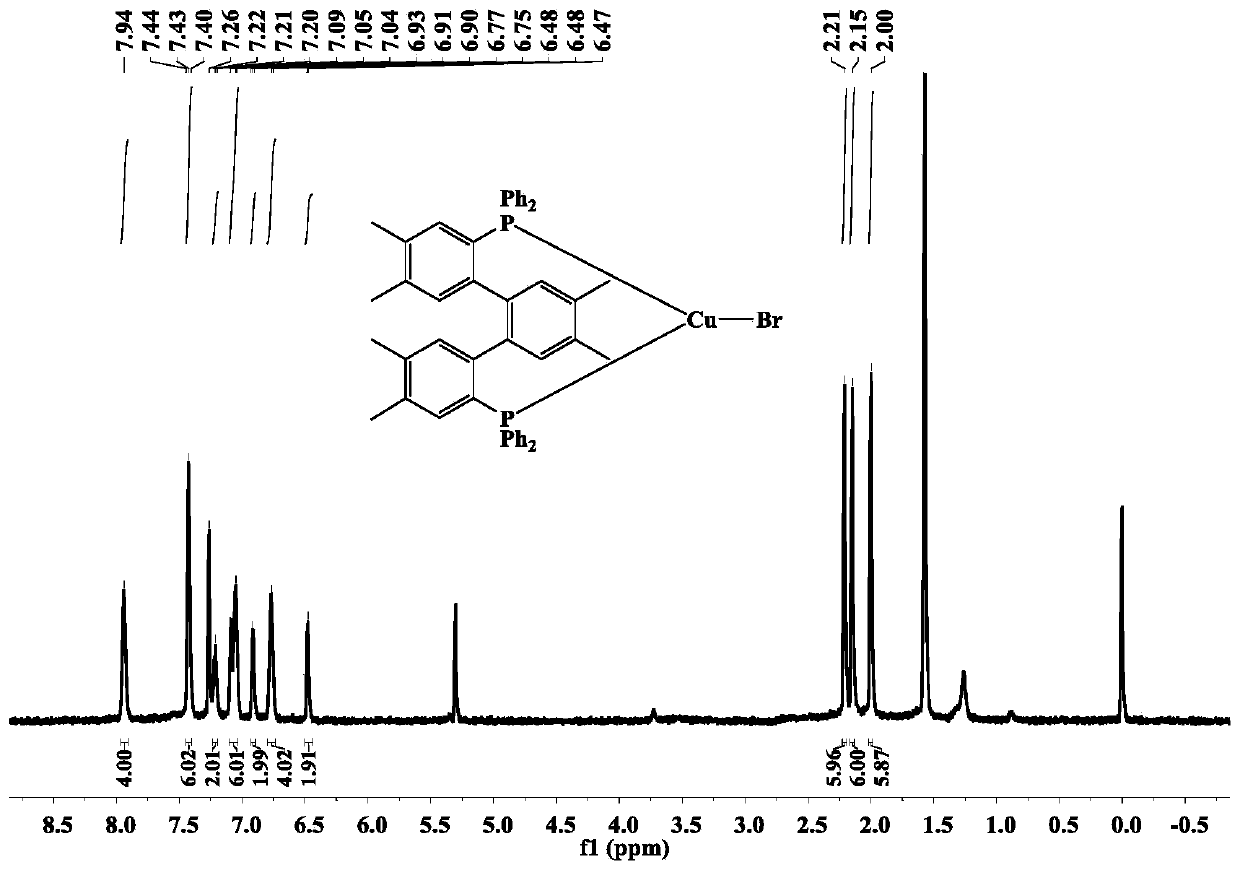
[0053] The terphenyl bisphosphine tricoordinate cuprous bromide complex (complex 2) of the present embodiment is prepared by the following method, including the following steps:
[0054] The dbdp (143 mg, 0.21 mmol) synthesized in Example 1 and cuprous bromide (0.21 mmol) were added to 20 mL of dichloromethane, reacted for 5 h, filtered, and the solvent was suspended under reduced pressure to obtain a pale yellow-green solid. Then the light yellow-green solid was dissolved in a mixed solvent of 10 mL of dichloromethane / ethanol (V:V=3:1), and a colorless crystal was finally obtained by solvent evaporation at room temperature, that is, the complex 2. The yield For: 78%, the relevant test data of the complex 2 are as follows:
[0055] 1 H NMR (600MHz, CDCl 3 ) test results are as follows: δ: 7.94(s,4H), 7.44~7.40(m,6H), 7.22~7.20(m,2H), 7.09~7.04(m,6H), 6.93~6.90(m,2H), 6.78~6.75(m,4H),6.48~6.47(m,2H),2.21(s,6H),2.15(s,6H),2.00(s,6H).
[0056] 31 P NMR (240M, CDCl 3 ) test ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com