A kind of method of solid-state ball milling synthetic Sorafenib
A ball-milling, solid-state technology, applied in the direction of organic chemistry, can solve the problem of no reports, and achieve the effects of easy operation, speeding up the reaction rate, and shortening the reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
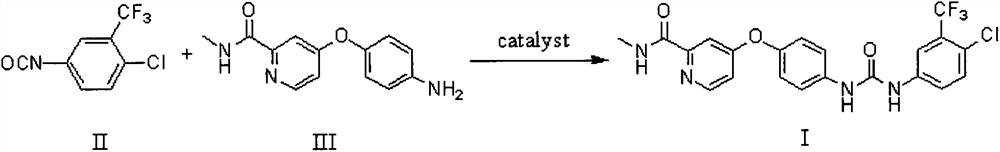
Embodiment 1
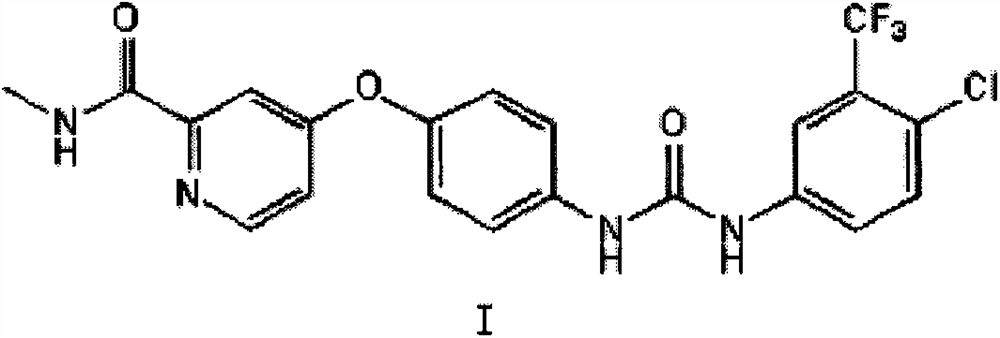
[0026] Preparation of 4-{4-[({[4-chloro-3-(trifluoromethyl)phenyl]amino}carbonyl)amino]phenoxy}-N-methylpyridine-2-carboxamide
[0027] In a 100mL polytetrafluoroethylene ball mill jar equipped with about 100g of zirconia grinding balls, add 0.50g (2.06mmol) of 4-(4-aminophenoxy)-N-methylpyridine-2-carboxamide, 4 - 0.46 g (2.06 mmol) of chloro-3-trifluoromethylphenyl isocyanate and 240 µl of acetonitrile (0.25 µl / mg solid compound). The rotating speed of the ball mill was set at 400r / min, and the reaction was stopped after 4h in a closed state of the tank. After the reaction was completed, the methanol-dissolved product was added into a ball mill, and evaporated to dryness by rotary evaporation, and purified to obtain 0.92 g of brown powder (yield 96%) with a purity of 99% (HPLC method). 1H NMR (600MHz, DMSO-d6) δ: 2.79(d, J=4.9Hz, 3H), 7.18(m, 3H), 7.38(d, J=2.6Hz, 1H), 7.62(m, 4H), 8.13 (d, J=2.5Hz, 1H), 8.51(d, J=5.6Hz, 1H), 8.78(q, J=4.8Hz, 1H), 9.02(s, 1H), 9.24(s, 1H)....
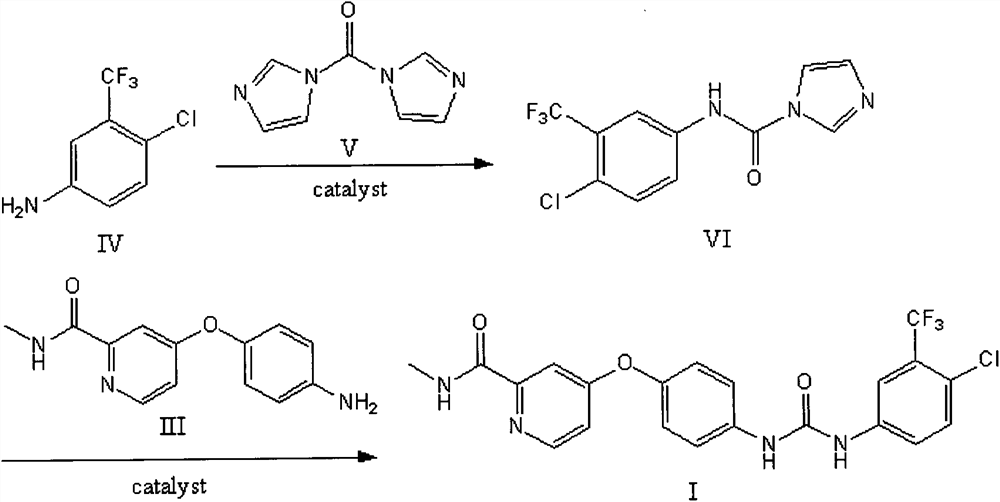
Embodiment 2
[0029] Preparation of N-(4-chloro-3-trifluoromethylphenyl)-1H-imidazol-1-amide
[0030] In a 100mL polytetrafluoroethylene ball mill jar equipped with about 100g of zirconia grinding balls, add 1g (5.113mmol) of 4-chloro-3-trifluoromethylaniline, 0.83g (5.118mmol) of N,N'-carbonyldiimidazole mmol) and 460 μl of acetonitrile (0.25 μl / mg solid compound). The rotational speed of the ball mill was set at 400r / min, followed by TLC until the reaction was complete, and the reaction was stopped after 5h in a closed state of the tank. After completion, the reaction mixture was vigorously stirred with water, filtered with suction, the filtrate was discarded, and dried overnight to obtain 1.18 g of white powder (yield 80%).
Embodiment 3
[0032] Preparation of 4-{4-[({[4-chloro-3-(trifluoromethyl)phenyl]amino}carbonyl)amino]phenoxy}-N-methylpyridine-2-carboxamide
[0033] In a 100mL polytetrafluoroethylene ball mill jar equipped with about 100g of zirconia grinding balls, add 0.12g (0.49mmol) of 4-(4-aminophenoxy)-N-methylpyridine-2-carboxamide, N -(4-chloro-3-trifluoromethylphenyl)-1H-imidazole-1-amide 0.14g (0.48mmol), potassium carbonate 0.14g (1.01mmol) and dichloromethane 65μl (0.25μl / mg solid compound ). The rotating speed of the ball mill was set at 400r / min, and the reaction was stopped for 8 hours in a closed state of the tank. Add methanol lysate in the ball mill jar after reaction finishes, and liquid is transferred in the pear-shaped bottle, heating makes dissolving completely, and rotary evaporation is to dryness, purifies brown powder 0.17g (yield 72%), and purity 99% (HPLC Law). 1H NMR (500MHz, DMSO-d6): δ2.81(d, J=4.9Hz, 3H), 7.18(m, 3H), 7.40(d, J=2.6Hz, 1H), 7.66(m, 4H), 8.14 (d, J = 2.5Hz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com