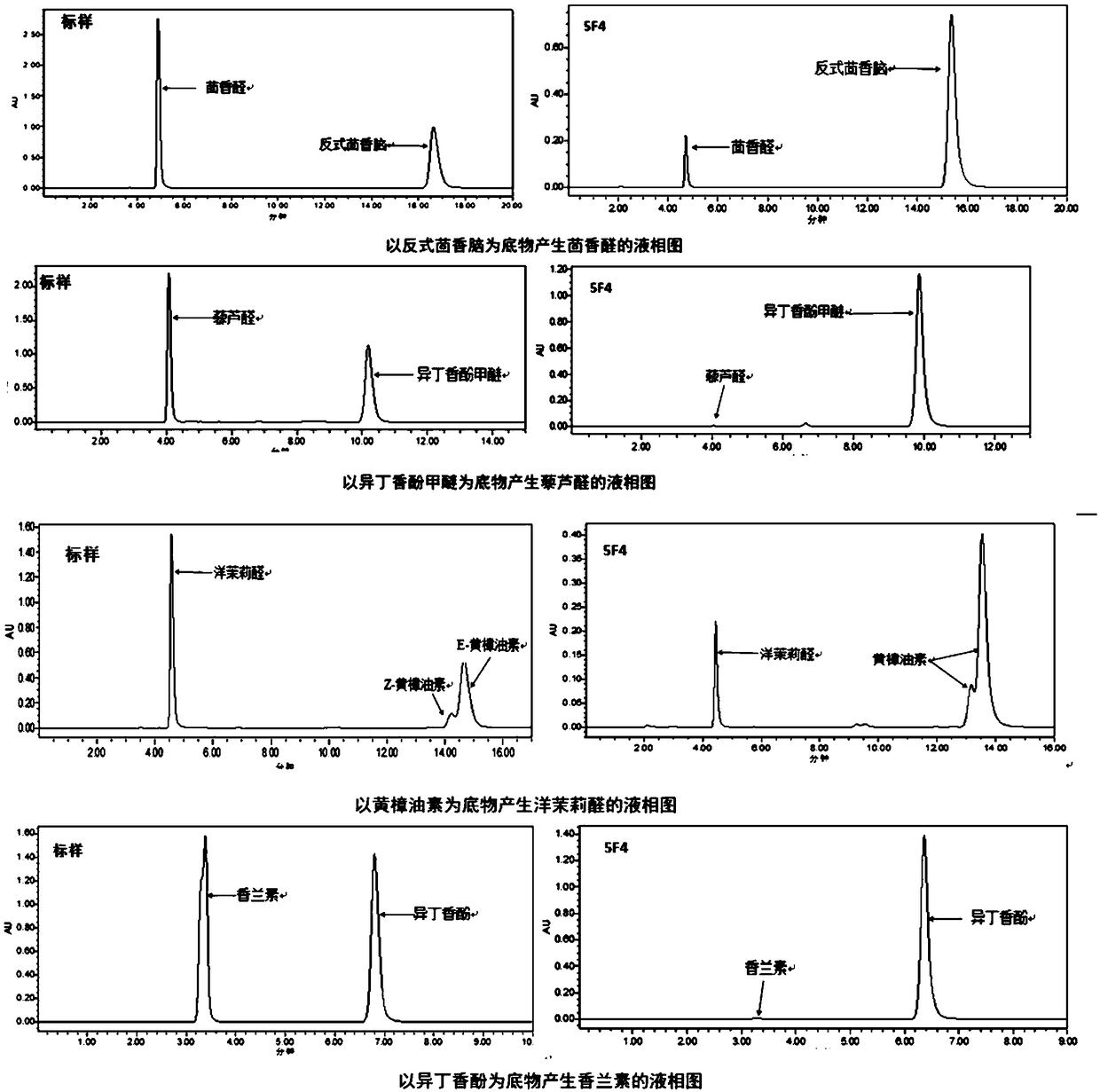
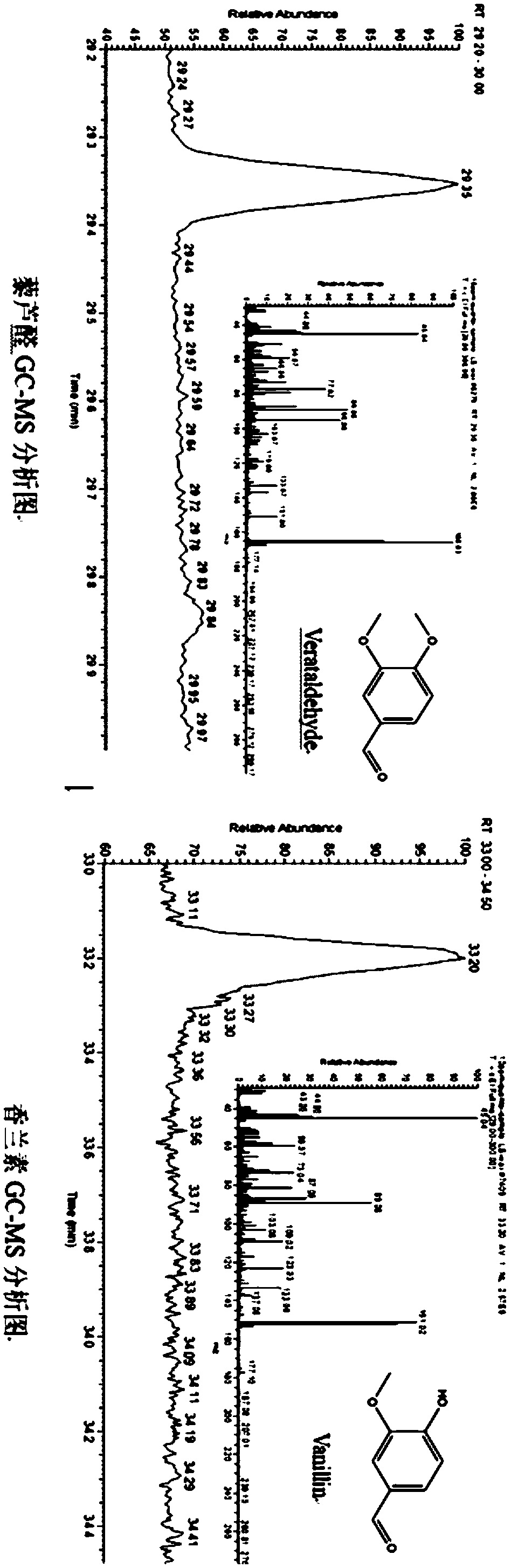
Mutant and mutant strain of trans-anethole oxygenase
A technology of trans-anethole and brain oxygenase, applied in the field of bioengineering, can solve the problem of low catalytic efficiency and achieve the effects of good application potential, cost reduction and wide adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Establishment of high-throughput screening methods:
[0028] (1) Effect of different alkali solutions on color development
[0029] Measure 1mol·L respectively -1 The influence of NaOH-50% ethanol solution and KOH-50% ethanol solution on color development, record OD 470 and stability (OD within 1h 470 The ratio of the absolute value of the maximum difference to the current use value), as shown in Table 1.
[0030] Table 1 The influence of different alkali solutions on color development
[0031] lye
NaOH
KOH
OD 470
2.86
1.814
stability
0.016
0.055
[0032] The results show that the OD using NaOH-50% ethanol solution 470 Higher response and better stability.
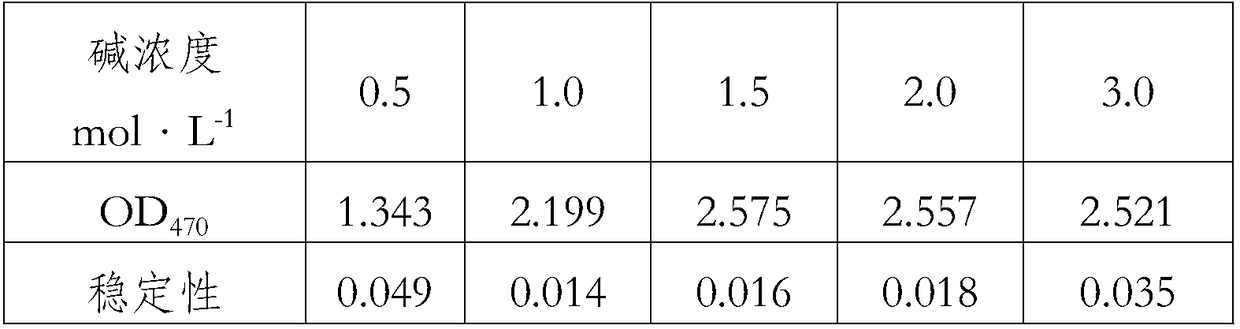
[0033] (2) Effect of different alkali concentrations on color development
[0034] Measure 0.5~3.0mol·L respectively -1 The effect of NaOH on color development, record OD 470 and stability, as shown in Table 2.
[0035] Table 2 Effects of different alkali c...
Embodiment 2
[0050] Taking the synthetic product jasmonal as an example, take 50 μL of jasmonal standard solution (concentration is 0-1000 mg L -1 ), add 50~300μL of 2,4-dinitrophenylhydrazine developer, react at 10~40℃ for 5~40min, add 0.5~3mol·L -1 NaOH-(0~80%) ethanol solution, reacted at 10~40°C for 5~60min, took it out, and measured its absorbance value OD by microplate reader 470 , with the jasminal concentration as the abscissa, and the OD470 value as the ordinate, draw a standard curve, and obtain the calculation formula as y=0.00496x+0.09445, R 2 is 0.9995, and the detection concentration range of jasmonal is 0~700mg·L -1 . This method can be used as a high-throughput screening method.
Embodiment 3
[0052] The establishment of the tao mutation library:
[0053] (1) Error-prone PCR to establish a tao mutation library
[0054] According to the trans-anethole oxygenase gene in Pseudomonas putida JYR-1, tao was synthesized and connected to PGEX-6P-1 to obtain the recombinant plasmid PGEX-6P-1-tao, which was used as a template to design error-prone PCR primers taoF, taoD, set Mg 2+ Concentration, Mn 2+ Concentrations are 7~10mmol·L -1 And 30~250μmol·L -1 , error-prone PCR using common rTaq polymerase. Primers are as follows:
[0055] taoF: ttccaggggcccctgggatccGGATCCATGGAGGACATCATGC
[0056] taoD: gtcacgatgcggccgctcgagCTCGAGTCAGTTAGTCCTCAAGTCG
[0057] The PCR program was set as follows:
[0058]
[0059] Use nucleic acid gel electrophoresis to detect PCR products, purify and recover the PCR products, and then use a one-step cloning kit to connect to the linear vector PGEX-6P-1, and transform into E.coli BL21 (DE3) super competent, spread culture , picked a single ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com