Preparation and application of cytomembrane biomimic lipoprotein targeted nanometer drug delivery system
A nano-drug delivery system and cell membrane technology are applied in the field of preparation and application of a cell membrane biomimetic lipoprotein targeted nano-drug delivery system, which can solve problems such as limitations, achieve high safety, reduce identification and clearance, and improve bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
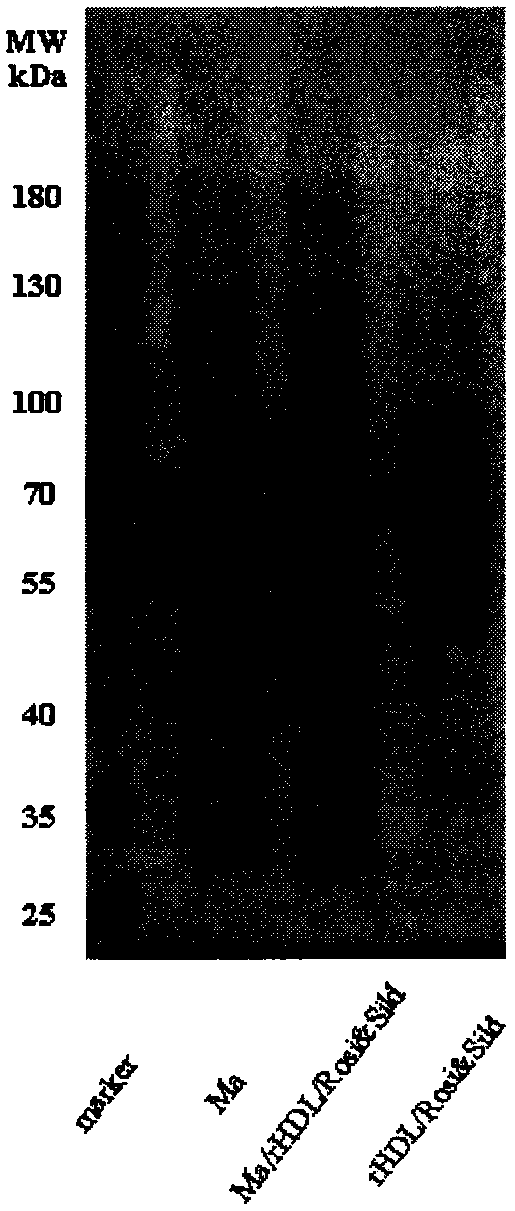
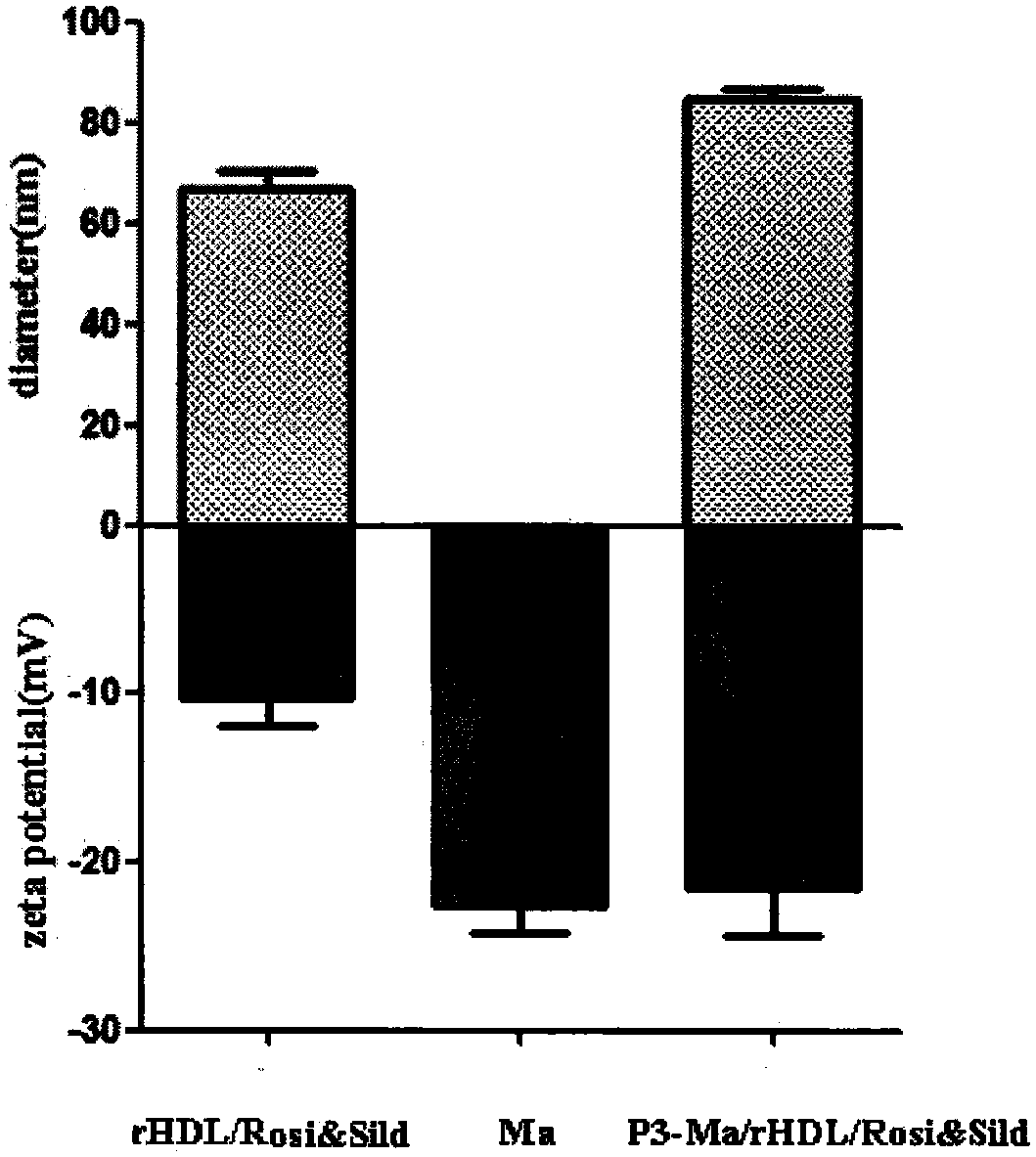
[0041] 1. Preparation of recombinant high-density lipoprotein drug-loaded core (rHDL / Rosi&Sild)
[0042] Weigh a certain amount of phospholipids, cholesterol, rosiglitazone and sildenafil, mix and dissolve them in an organic solvent, spin-evaporate under reduced pressure in a water bath at 37°C to form a thin film, then dry in vacuum overnight to remove excess organic solvent, and then add Appropriate amount of pure water was completely hydrated in a 40°C water bath, and ultrasonically dispersed to obtain an opalescent nanosuspension; add a certain amount of ApoA-I phosphate buffer (PBS, pH 7.4) and stir overnight, and deionized water Dialyzed for 12 hours to remove free ApoA-I to obtain the recombinant high-density lipoprotein drug-loaded inner core. The coumarin and the double drug were added together, and the above steps were repeated to obtain fluorescently labeled rHDL / Rosi&Sild.
[0043] Wherein the preparation process conditions of recombinant high-density lipoprotein ...
Embodiment 7-10
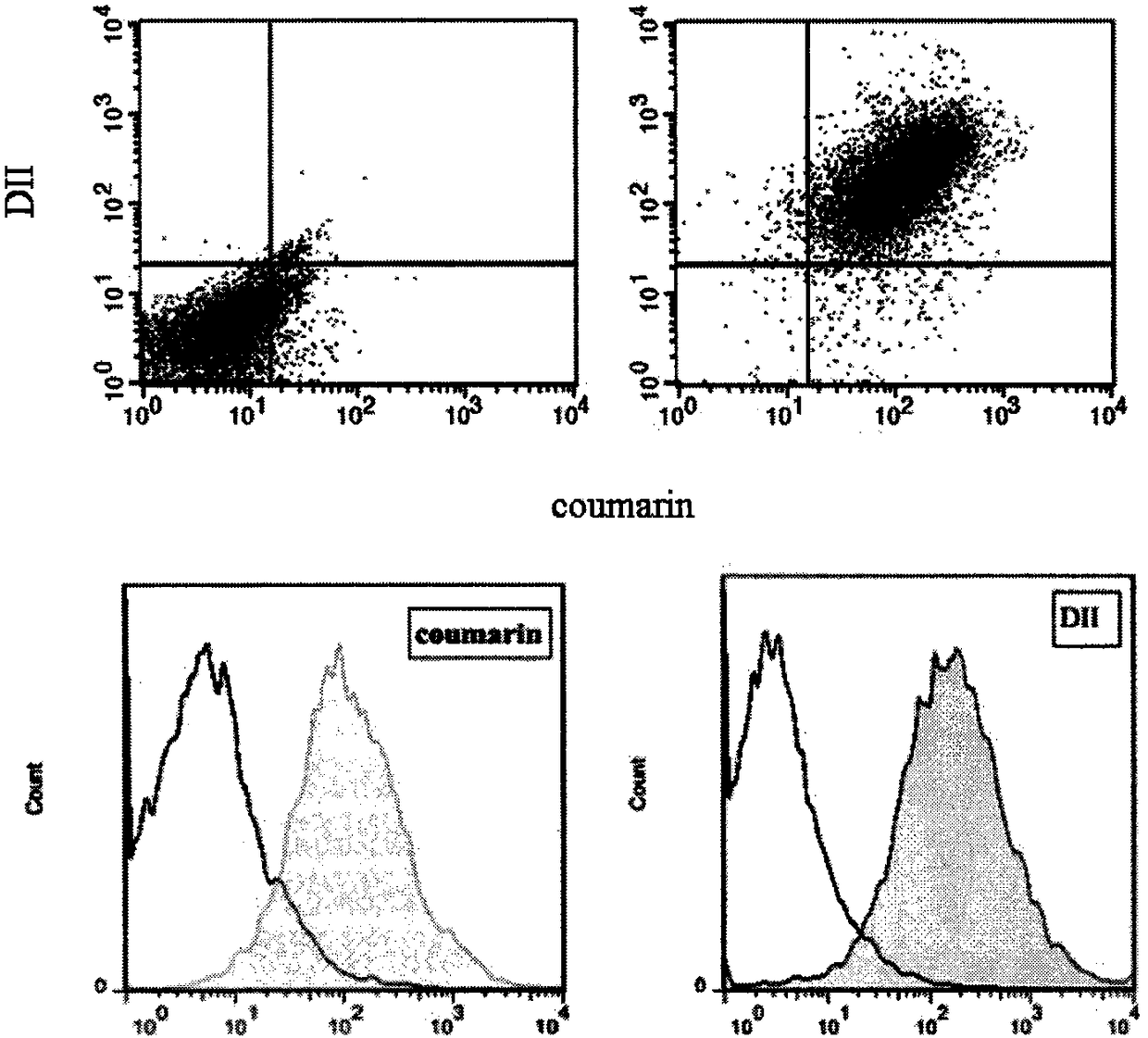
[0048] 2. Extraction of macrophage membrane (Ma)
[0049] The culture medium in the macrophage culture flask was discarded, the macrophages were blown off after washing with PBS solution (pH 7.4) for 3 times, and the supernatant was removed by centrifugation at 1000 rpm. Add cell lysate (1mM sodium bicarbonate, 0.2mM ethylenediaminetetraacetic acid and 1mM serine protease inhibitor) to the pellet, lyse at 4°C overnight, then sonicate the probe for 10min, centrifuge at 3200×g for 5-10min, discard the pellet, and The supernatant was centrifuged at 4°C at 18,000×g for 45-60 min, discarded, and the pellet was resuspended in PBS solution (pH 7.4) to obtain a macrophage membrane suspension. The DiI PBS solution was added to the prepared macrophage membrane mixture and incubated for 35 minutes, and dialyzed against deionized water for 12 hours to obtain a fluorescently labeled macrophage membrane suspension.
[0050] Wherein, the extraction process conditions of macrophage membrane ...
example 16-19
[0060] 4. Preparation of P3 peptide-modified cell membrane biomimetic recombinant high-density lipoprotein drug-loaded nanoparticles (P3-Ma / rHDL / Rosi&Sild)
[0061] The PBS solution (pH 7.4) of the P3 peptide-stearic acid conjugate was mixed with the cell membrane biomimetic recombinant high-density lipoprotein drug-loaded nanoparticle suspension in a certain proportion, incubated at 37°C for 60 minutes, and then dialyzed with deionized water for 12 hours. The free peptide is removed to obtain the P3 peptide-modified cell membrane biomimetic recombinant high-density lipoprotein drug-loaded nanoparticle. .
[0062] Among them, the preparation process conditions of the P3 peptide-modified cell membrane biomimetic recombinant high-density lipoprotein drug-loaded nanoparticles in Examples 16-19 are shown in Table 4:
[0063] Table 4 Process conditions for the preparation of P3 peptide-modified cell membrane biomimetic recombinant high-density lipoprotein drug-loaded nanoparticles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com