Lithium manganate positive electrode material for lithium ion batteries and preparation method of lithium manganate positive electrode material
A battery lithium manganese oxide and positive electrode material technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of difficulty in meeting the requirements of long battery life of power lithium batteries, restrictions on large-scale industrial applications, and poor stability, and achieve Suppresses oxygen defects, improves structural stability, and avoids the effect of reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The present invention also provides a preparation method of lithium manganate cathode material for lithium ion battery, comprising the following steps:
[0038] providing a mixed powder comprising a lithium source, a manganese source and a dopant source; the dopant source comprises a silicon source, a zirconium source or a titanium source;
[0039] The molar ratio of lithium source, manganese source and dopant source in the mixed powder is 1: [1.9,2): (0,0.1];
[0040] (2) pre-sintering and re-sintering the mixed powder obtained in the step (2) successively to obtain LiMn 2-x m x o 4 Nucleus; the pre-sintering temperature is 400-450°C, and the pre-sintering time is 4-6h; the re-sintering temperature is 750-825°C, and the re-sintering time is 12-18h; the M is Si , Zr or Ti;
[0041] (3) LiMn obtained in said step (2) 2-x m x o 4 In situ synthesis of fast ion conductor cladding on the surface of the core to obtain primary core-shell materials;
[0042] (4) In-situ...
Embodiment 1
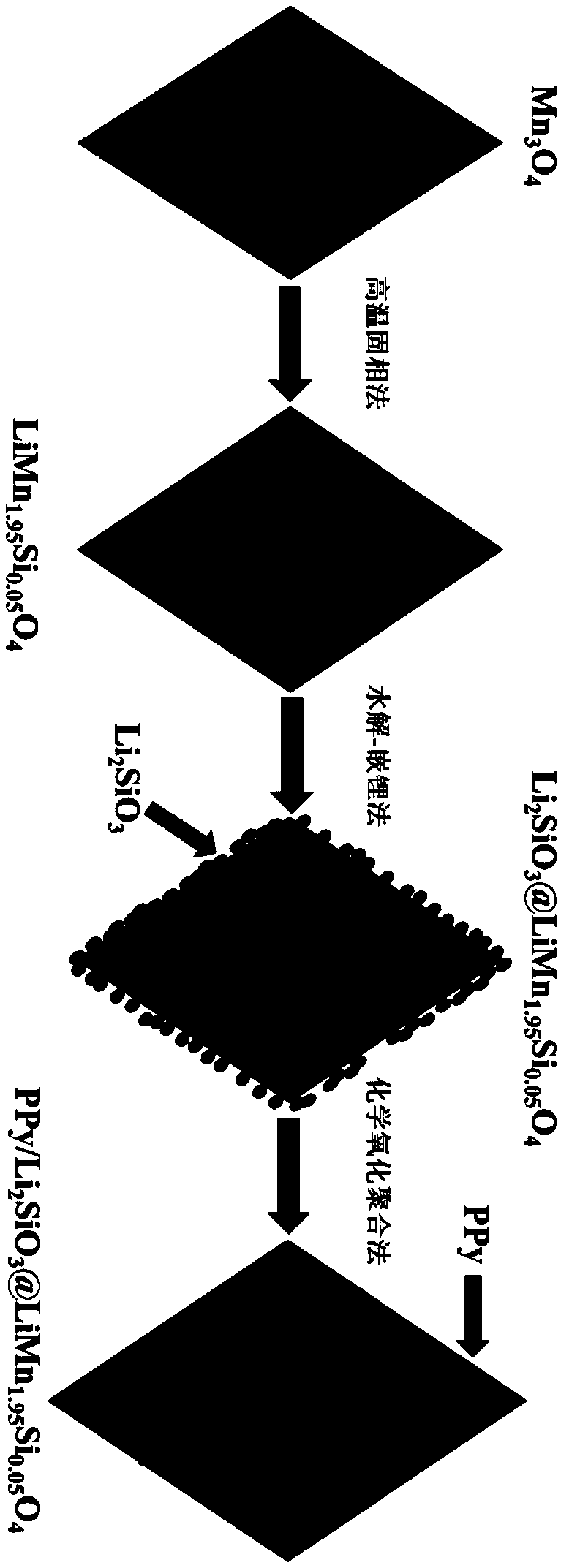
[0098] according to figure 1 The flow chart shown prepares lithium manganate cathode material for lithium ion batteries:
[0099] The absolute ethanol solution of lithium carbonate, manganese tetraoxide and ethyl orthosilicate was mixed according to the molar ratio of Li, Mn and Si of 1.05:1.95:0.05, and wet ball milled for 3 hours. After ball milling, the obtained mixed slurry was transferred to an evaporating dish for drying. Finally, grind the dried mixture evenly and place it in a muffle furnace for pre-calcination at 450°C for 4 hours, then grind it again and place it in a muffle furnace for final firing at 800°C for 18 hours to obtain LiMn 1.95 Si 0.05 o 4 .
[0100] 10g of LiMn 1.95 Si 0.05 o 4 Ultrasonic dispersion in a mixed solution of absolute ethanol and deionized water, then dissolve 0.35g tetraethyl orthosilicate in absolute ethanol, and then mix the absolute ethanol solution of tetraethyl orthosilicate with the mixed solution of nuclei Afterwards, ammonia ...
Embodiment 2
[0106] The absolute ethanol solution of lithium hydroxide, electrolytic manganese dioxide and nano-titanium dioxide was mixed according to the molar ratio of Li, Mn and Ti of 1.05:1.95:0.05, and wet ball milled for 3 hours. After ball milling, the obtained mixed slurry was transferred to an evaporating dish for drying. Finally, grind the dried mixture evenly and place it in a muffle furnace for pre-calcination at 400°C for 6 hours, then grind it again and place it in a muffle furnace for final firing at 780°C for 15 hours to obtain LiMn 1.95 Ti 0.05 o 4 .
[0107] According to the molar ratio of lanthanum, strontium, manganese and cobalt as 0.7:0.3:0.7:0.3, dissolve lanthanum nitrate, strontium nitrate, manganese nitrate and cobalt nitrate in deionized water at 50°C, and add lemon Acid acts as a chelating agent, and ammonia water is added dropwise to adjust the pH of the solution to 3. Then, the solution was heated to 70°C, and 10 g of LiMn was added 1.95 Mg 0.05 o 4 , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
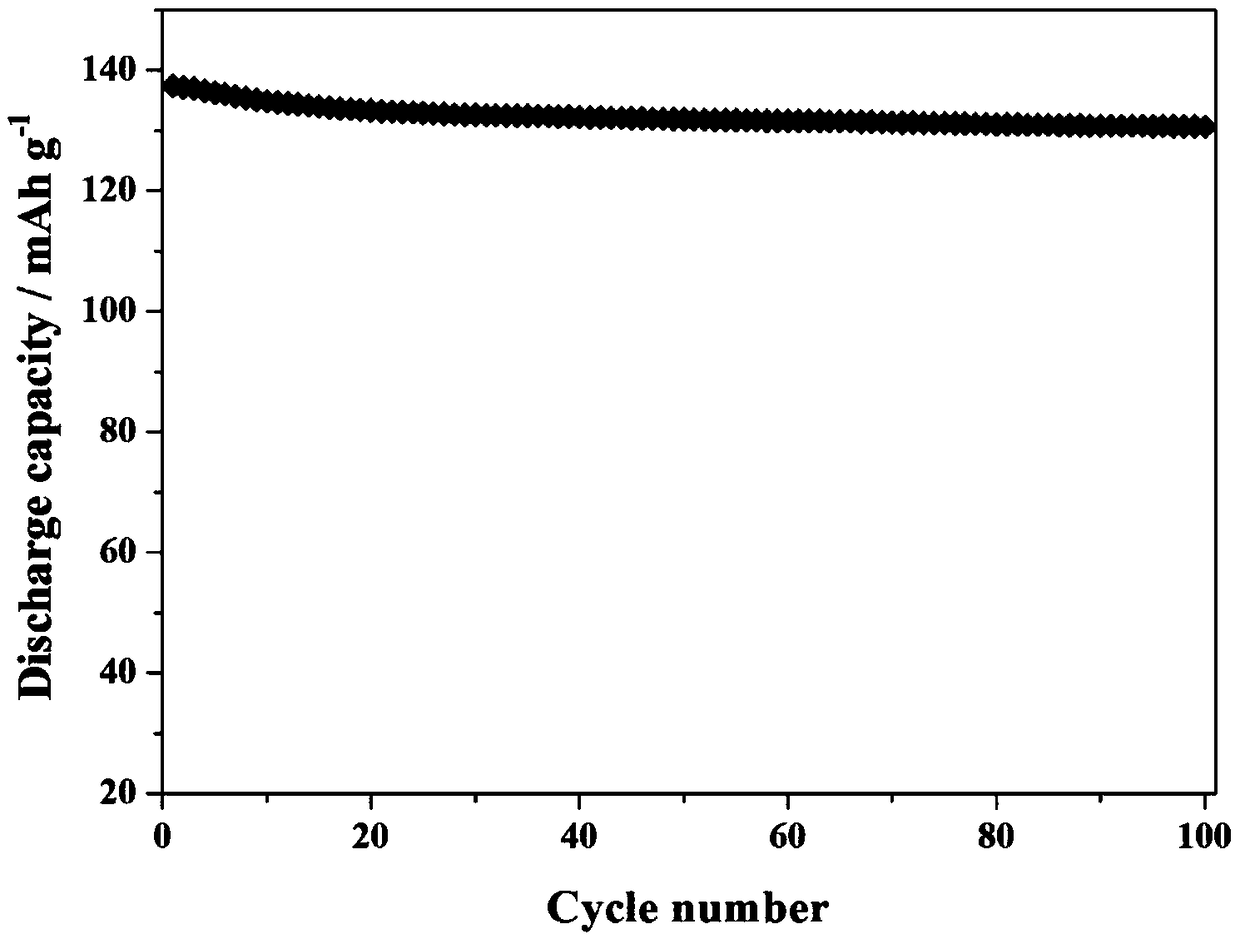
| retention rate | aaaaa | aaaaa |
| retention rate | aaaaa | aaaaa |
| retention rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com