A kind of tertiary amino amphoteric ion exchange membrane and preparation method thereof
A technology of amphoteric ions and exchange membranes, applied in fuel cells, electrical components, electrochemical generators, etc., can solve the problems of high vanadium ion permeability and large surface resistance, achieve excellent battery performance, improve stability, and improve membrane The effect of vanadium barrier properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
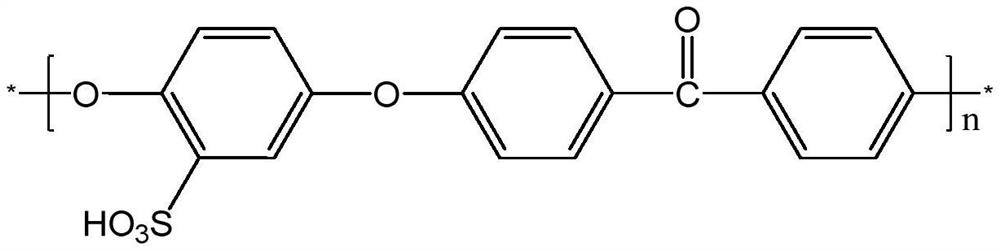
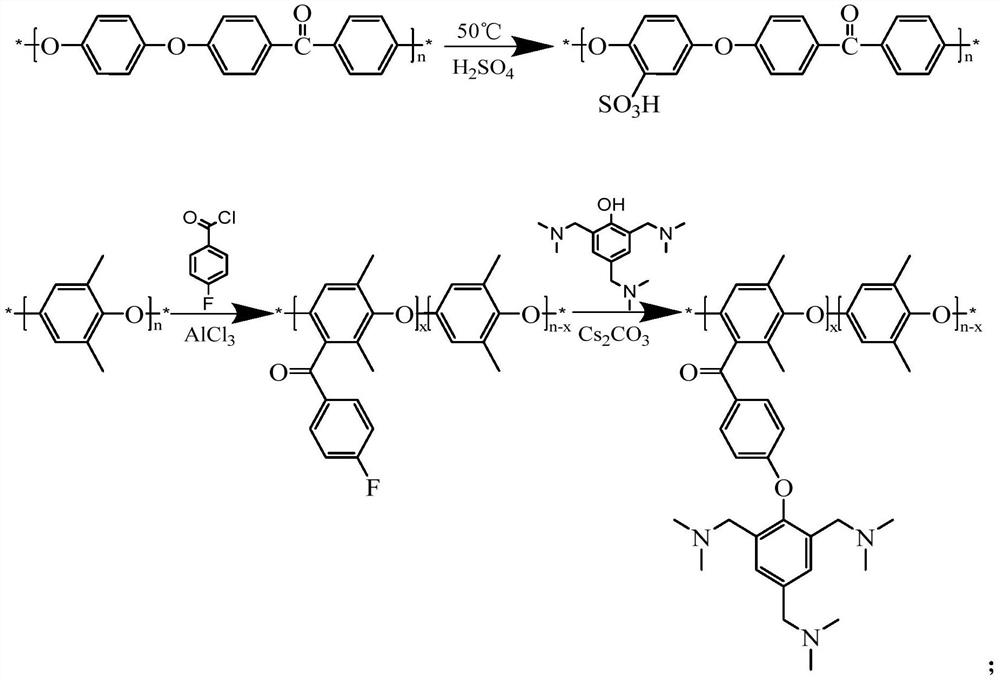
[0028] Under the condition of an ice-water bath, 5 g of polyether ether ketone (PEEK) was dissolved in 100 ml of concentrated sulfuric acid, and vigorously stirred. When the polymer was completely dissolved, the reaction temperature was increased to 50°C, and the reaction was carried out for 7 hours. After the reaction was completed, the reaction solution was poured into ice water in a stirring state, and a sulfonated polyetheretherketone (SPEEK) polymer with a sulfonation degree of 78% was precipitated. The product was washed with deionized water until neutral, and completely dried under vacuum environment;
[0029] Dissolve 0.15 g of the synthesized SPEEK in 4 ml of DMF and cast at 50°C for 24 hours to form a film. The membrane was soaked in deionized water for 12 h at room temperature to remove impurities. Then, the membrane was soaked in acid for 12h to make it fully ion exchanged. The membrane is then soaked in deionized water to remove excess acid. The SPEEK film is ...
Embodiment 2
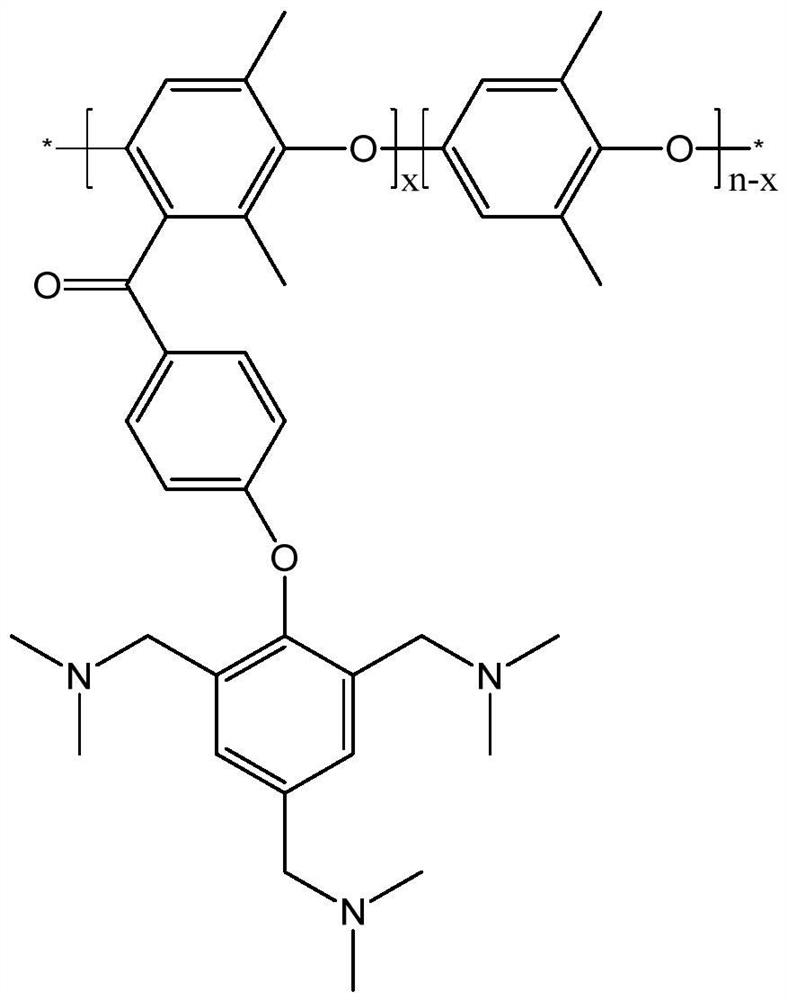
[0031] Under stirring state, 3g PPO is dissolved in 25ml dichloromethane to make PPO solution, then in another three-necked flask, add 25ml dichloromethane, add 2.4g anhydrous aluminum chloride and vigorously stir under nitrogen protection, in 1.83ml of 4-fluorobenzoyl chloride was slowly added under ice-water bath conditions. Then the PPO solution prepared before was added into this mixed solution, and reacted at 60° C. for 10 h. After the reaction is completed, the reaction solution is poured into a large amount of ethanol, and the precipitate is an acylated PPO polymer with a substitution degree of 50%. Wash the polymer repeatedly with water and ethanol, and dry it in vacuum;
[0032] Dissolve 1g of acylated PPO synthesized above in 30ml of dimethylacetamide (DMAC), then add 1g of 2,4,6-tris(dimethylaminomethyl)phenol and 1.6g of cesium carbonate, and react at 110°C 10h. During the reaction, nitrogen gas was bubbled through the solution to facilitate the removal of water...
Embodiment 3
[0035] The main chain polymers SPEEK and PPO-TA were prepared as described in Example 1 and Example 2. 0.1425g SPEEK and 0.0075g PPO-TA were dissolved in 4ml DMF respectively, and cast at 50°C for 24h to form a film. The membrane was soaked in deionized water for 12 h at room temperature to remove impurities. Then, the membrane was soaked in acid for 12h to make it fully ion exchanged. The membrane is then soaked in deionized water to remove excess acid. Make SPEEK / PPO-TA (5%) membrane, this membrane water absorption rate is 41.8%, and surface resistance is 0.354Ωcm , vanadium ion permeability is 0.82 * 10 -8 cm 2 the s -1 , in the all-vanadium redox flow battery cell test, at 80mA·cm -2 At current density, CE is 90.04%, VE is 91.27%, and EE is 82.18%. At 1.5M VO 2 + / 3M H 2 SO 4 The mass loss after soaking in the solution for 45 days was 14%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com