Preparation method and application of hydrotalcite-based CoMnFe composite metal oxide denitration catalyst
A denitrification catalyst and composite metal technology, which is applied in the field of preparation of hydrotalcite-based CoMnFe composite metal oxide denitration catalysts, can solve problems such as restricting water resistance and sulfur resistance, and achieve excellent water resistance and sulfur resistance, strong thermal stability, The effect of good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
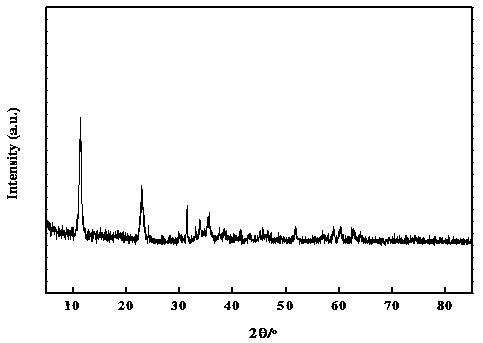
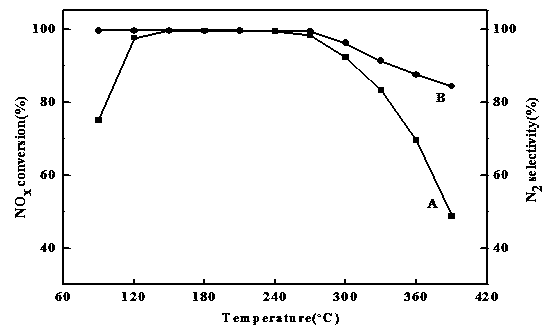
Image
Examples
Embodiment 1
[0033] (1) Weigh 8.80 g of cobalt nitrate hexahydrate, 5.050 g of ferric nitrate nonahydrate, measure 1.8 mL of manganese nitrate solution with a mass fraction of 50%, add them to the beaker in turn, and add deionized water to the beaker to the mark line of 100 mL place, and stirred with a magnetic stirrer for 10 min to dissolve it completely, and transferred the prepared solution to a 250 mL hanging bottle infusion set for use;
[0034] (2) Weigh 4.00 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to dissolve it completely. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00 mol L -1 sodium hydroxide aqueous solution, and then transfer it to a 250 mL hanging bottle infusion set for use;
[0035] (3) Sodium citrate aqueous solution: Weigh 0.370 g of sodium citrate solid and place it in a beaker, add 5 mL of deionized water to prepare a sodium citrate aqueous solution...
Embodiment 2
[0040] (1) Weigh 8.20 g of cobalt nitrate hexahydrate, 5.050 g of ferric nitrate nonahydrate, measure 2.2 mL of manganese nitrate solution with a mass fraction of 50%, add them to the beaker in turn, and add deionized water to the beaker to the mark line 100 mL and stir it with a magnetic stirrer for 10 min to dissolve it completely, then transfer the prepared solution to a 250 mL hanging bottle infusion set for use;
[0041] (2) Weigh 4.00 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to dissolve it completely. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00 mol L -1 sodium hydroxide aqueous solution, and then transfer it to a 250 mL hanging bottle infusion set for use;
[0042] (3 Sodium citrate aqueous solution: Weigh 0.370 g of sodium citrate solid and place it in a beaker, add 5 mL of deionized water to prepare a sodium citrate aqueous solution, place it in...
Embodiment 3
[0047] (1) Weigh 7.300 g of cobalt nitrate hexahydrate, 5.050 g of ferric nitrate nonahydrate, measure 2.9 mL of manganese nitrate solution with a mass fraction of 50%, add them to the beaker in turn, and add deionized water to the beaker to the mark line of 100 mL place, and stirred with a magnetic stirrer for 10 min to dissolve it completely, and transferred the prepared solution to a 250 mL hanging bottle infusion set for use;
[0048] (2) Weigh 4.000 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to completely dissolve it. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00 mol L -1 sodium hydroxide aqueous solution, and then transfer it to a 250 mL hanging bottle infusion set for use;
[0049] (3) Sodium citrate aqueous solution: Weigh 0.370 g of sodium citrate solid and place it in a beaker, add 5 mL of deionized water to prepare a sodium citrate aqueous soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com