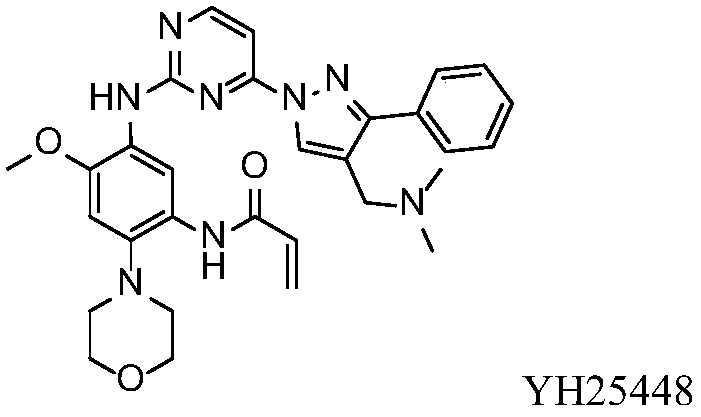
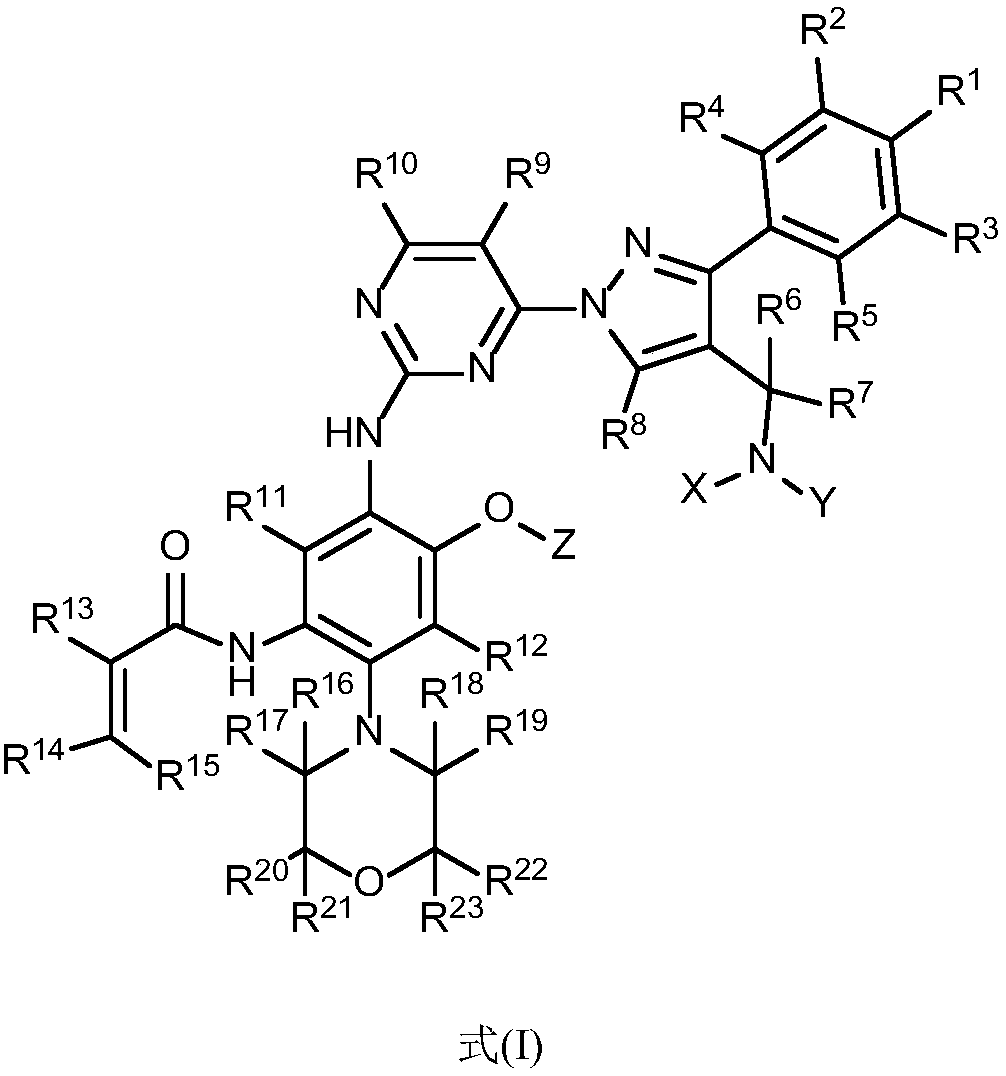
Aminopyrimidine compound, composition containing same and application thereof
一种氨基嘧啶、化合物的技术,应用在氨基嘧啶类化合物及包含该化合物的组合物领域,能够解决限制应用范围、病人依从性差、副作用治疗成本等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Embodiment 1N-(5-((4-(4-((dimethylamino)methyl-d 2 )-3-phenyl-1H-pyrazol-1-yl-5-d)pyrim Preparation of pyridin-2-yl)amino)-4-methoxy-2-morpholinophenyl)acrylamide (compound T-1).
[0123]
[0124] Concrete synthetic steps are as follows:
[0125]
[0126]
[0127] Step 1 Synthesis of compound 2.
[0128] Add 4-chloro-2-methylthiopyrimidine (compound 1) (7.25mL, 62.3mmol) and 95% ethanol (100mL) to a 250mL single-necked flask equipped with magnetic stirring in sequence, stir to dissolve, cool to 0°C, Ammonium molybdate tetrahydrate (2.18g, 1.87mmol) in hydrogen peroxide (30%, 14.4mL, 187mmol) was slowly added dropwise, and after the drop was completed, the temperature was raised to room temperature, and the reaction was stirred overnight under nitrogen.
[0129] Evaporate most of the organic solvents under reduced pressure, add water (200mL), extract with dichloromethane (70mLx3), combine the organic phases, wash with saturated brine, dry over anhydrous sod...
Embodiment 2
[0162] Example 2N-(5-((4-(4-((dimethylamino)methyl)-3-(phenyl-d 5 )-1H-pyrazol-1-yl)pyrimidine- Preparation of 2-yl)amino)-4-methoxy-2-morpholinophenyl)acrylamide (compound T-2).
[0163]
[0164] Concrete synthetic steps are as follows:
[0165]
[0166]
[0167] Step 1 Synthesis of Compound 18.
[0168] Add benzene-d to a 50mL three-necked flask equipped with magnetic stirring and condenser 6 (Compound 17, 3.0g, 35.65mmol) and carbon disulfide (15mL), stir well, add AlCl at room temperature 3 (10.46g, 78.43mmol), slowly heated to a slight boil (47°C) under nitrogen, slowly added acetic anhydride (2.91g, 28.52mmol) dropwise, and kept stirring for 1h.
[0169] Cool to room temperature, pour concentrated hydrochloric acid (3.5mL) into ice water (50g), stir for 10 minutes, separate the water phase, extract with ethyl acetate (30mLx3), combine the organic phases, wash with saturated brine, and dry over anhydrous sodium sulfate , filtered, concentrated, and passed t...
Embodiment 3
[0191] Example 3N-(5-((4-(4-((dimethylamino)methyl)-3-phenyl-1H-pyrazol-1-yl)pyrimidin-2-yl)ammonia Base)-4-(methoxy-d 3 )-2-Morpholinophenyl)acrylamide (Compound T-3).
[0192]
[0193] Concrete synthetic steps are as follows:
[0194]
[0195]
[0196] Step 1 Synthesis of compound 27.
[0197] Add acetonitrile (30mL) and 2-hydroxy-4-fluoronitrobenzene (compound 26) (3.1g, 20mmol) to a 100mL single-necked flask equipped with a magnetic stirrer, stir to completely dissolve, add TsOMe-d 3 (4.0 g, 21.2 mmol), stirred overnight at room temperature under nitrogen. The insoluble solid was filtered off, the filter cake was washed with ethyl acetate, the filtrate was concentrated, and passed through a silica gel column to obtain 3.2 g of a white solid, with a yield of 92.0%. LC-MS(APCI):m / z=175.1(M+1) + .
[0198] Step 2 Synthesis of compound 28.
[0199] Add ethanol (30mL) and compound 27 (3.2g, 18.4mmol) to a 100mL single-necked flask equipped with a magnetic sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com