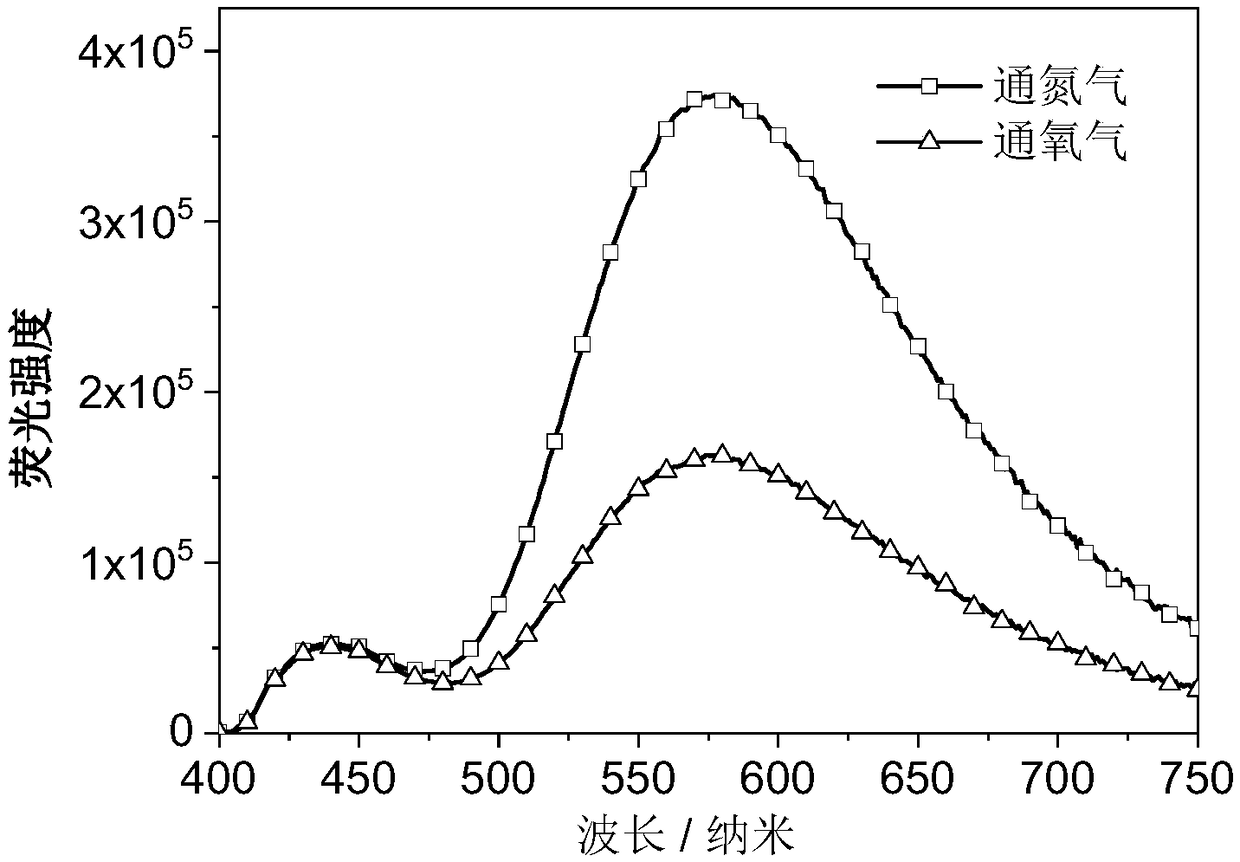
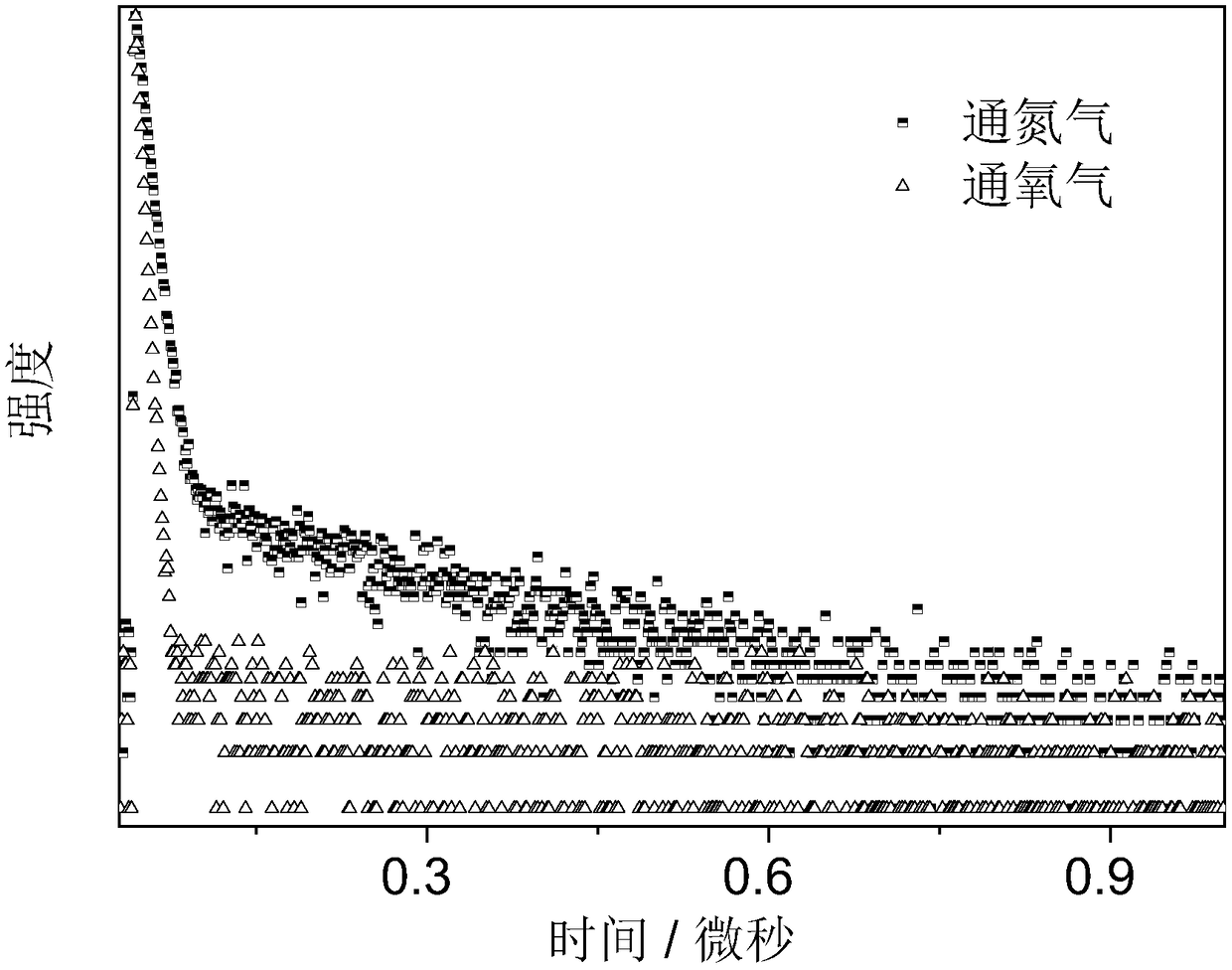
Luminescent material containing phenothiazine diphenyl ketone, compounding method and application thereof
A phenothiazine benzophenone, luminescent material technology, applied in luminescent materials, material analysis by optical means, material analysis and other directions, can solve the problems of poor sensitivity, high detection limit, rough detection means, etc., to achieve accurate detection, Easy purification, high fluorescence quantum efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Synthesis of intermediate [4,4-difluorobenzophenone]
[0043]
[0044] Add 4-fluorobenzoyl chloride (5.00g, 31.5mmol) into a 250mL dry three-necked flask, then add fluorobenzene (7.58g, 78.8mmol), stir, add anhydrous aluminum chloride (12.6g, 94.1mmol), Raise the temperature to 40-50°C and react for 5-6 hours. After the reaction, add 50ml of dichloromethane into the three-neck flask to dissolve, slowly add dilute hydrochloric acid, and stir until there is no precipitation. Then the reaction solution was poured into a separatory funnel, extracted 3 times with dichloromethane, and washed 2-3 times with dilute hydrochloric acid until the aqueous layer became colorless. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried in a rotary evaporator to obtain 5.73 g of a white solid. Yield 83.3%.
[0045] (2) Synthesis of intermediate [4-fluoro-4'-phenothiazinylbenzophenone]
[0046]
[0047] Add phenothiazine (1.03 g, ...
Embodiment 2
[0055] (1) synthesis of target product embodiment 2
[0056]
[0057] Referring to step (2) of Example 1, 4-carbazolyl-4'-phenothiazinyl benzophenone was used instead of 4,4-difluorobenzophenone to synthesize 4-carbazolyl-4'-phenothiazinyl diphenone benzophenone. (52.5% yield) 1 H NMR (300MHz, CDCl 3 )δ8.16-8.14(d,2H),8.05-8.03(d,2H),7.92-7.90(d,2H),7.72-7.70(d,2H),7.53-7.26(m,11H),7.25, 7.21-7.10(t,5H).
Embodiment 3
[0059] (1) Synthesis of intermediate [1-bromo-4-phenothiazinylbenzene]
[0060]
[0061] Add phenothiazine (3.00g, 15.1mmol) and 1-bromo-4-iodobenzene (4.68g, 16.6mmol) into a 250mL three-neck flask, add an appropriate amount of DMF to dissolve, add a teaspoon of iodine under stirring with argon gas Cuprous chloride, 18-crown-6 and o-phenanthroline were stirred evenly, and then potassium carbonate (6.51g, 45.3mmol) was added, heated to reflux and stirred for 12h. Cool to room temperature, pour into cold water, extract 3 times with dichloromethane, wash 2 times with water, dry the organic layer with anhydrous sodium sulfate, and spin dry in a rotary evaporator. Then it was purified by silica gel column chromatography, and the eluent was a mixed solution of dichloromethane and n-hexane with a volume ratio of 1:3. 2.54 g of white solid was obtained, with a yield of 47.6%.
[0062] (2) Synthesis of intermediate [4-phenothiazinyl phenyl borate]
[0063]
[0064] Add 1-brom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com