Preparation method for carrier-free nano-drug based on natural pigment and application
A technology of natural pigments and nano-drugs, applied in the field of preparation of carrier-free nano-drugs, can solve the problems of poor water solubility of chemotherapeutic drugs, complex preparation of nano-carriers, and toxicity of metabolism and excretion, and achieves good anti-tumor effect and water-solubility effect. Good, less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation method of prodigiosin nanometers Accurately weigh 0.00324g of PG powder, dissolve in 1ml of methanol, ultrasonically dissolve, and configure a 10 mM solution; take different volumes of the above solutions and dissolve them in 100μL of methanol, and dissolve them in 1ml of methanol. Add dropwise to 1 mL of secondary water (double distilled water) (note: vortex during the dropping process), at this time, the concentration of PG in the solution is 10μM-640μM, after ultrasonication for 10min, dry methanol , that is, PG nanometer; and use the precipitation method to determine the drug loading capacity of PG molecules in the nanomedicine (Drug loading capacity). Table 1 shows the average particle size, PDI, potential and drug loading of different concentrations of PG prepared in this example.
[0039] Table 1 The average particle size, PDI, potential and drug loading of different concentrations of PG nanoparticles
[0040]
Embodiment 2
[0042] Accurately weigh 0.00324g of PG powder, dissolve it in 1mL of methanol, ultrasonically dissolve, and configure a 10 mM solution; take different volumes of methanol solution, and add dropwise to the solution containing 1 mL of secondary water (double distilled water) during the stirring process. ) (note: vortex during the dropping process), the concentration of PG in the solution is 160μM at this time, after ultrasonication for 10min, dry the methanol to get PG nanometer;
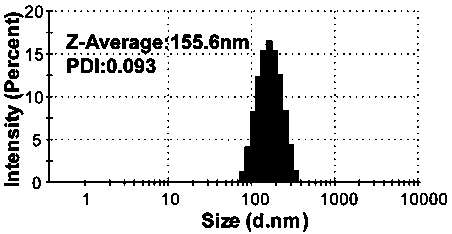
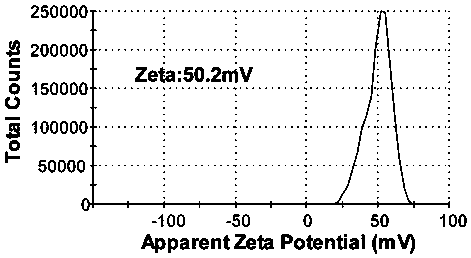
[0043] The PG nanoparticles prepared in this embodiment have a diameter of 155.6 and a potential of 50.2mV such as figure 1 and figure 2 shown.
Embodiment 3
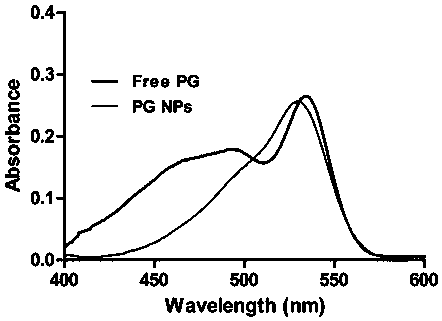
[0045] The PG nano-medicine prepared in Example 2 and the PG methanol solution of the same concentration were detected by ultraviolet-visible spectrophotometer, and the results showed that the nano-anticancer drug prepared by the present invention still had PG characteristic absorption peaks, such as image 3 As shown, it is proved that PG self-assembly of the present invention forms PG NPs anticancer nanomedicine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com