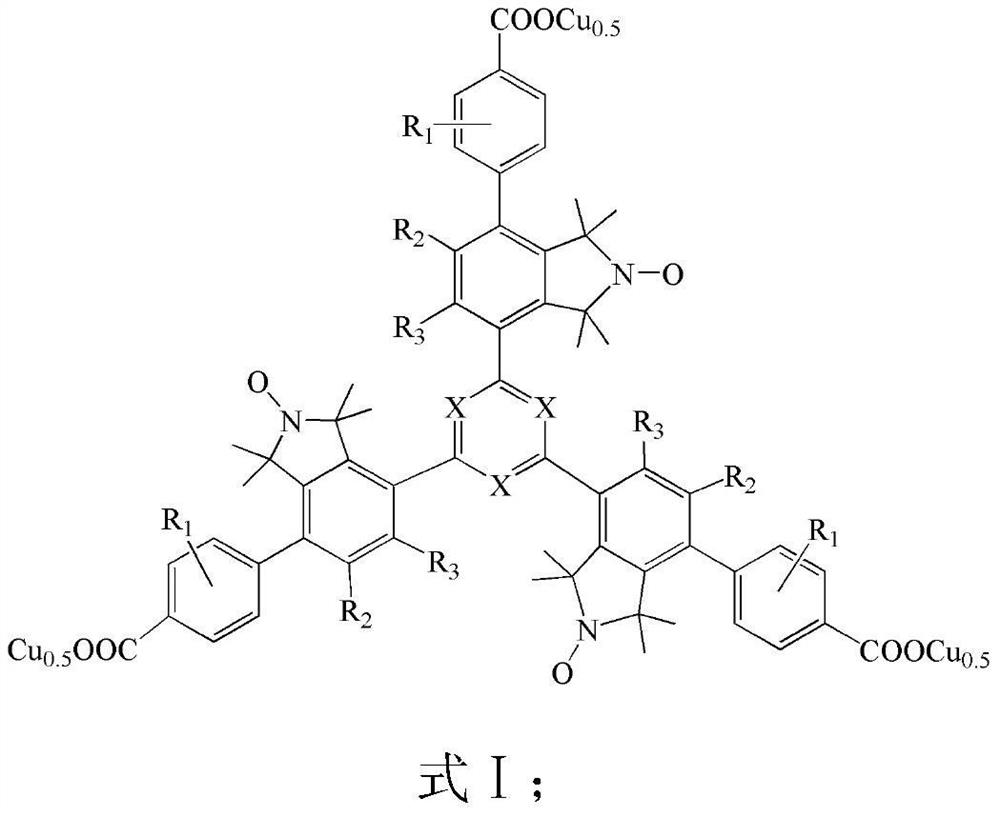
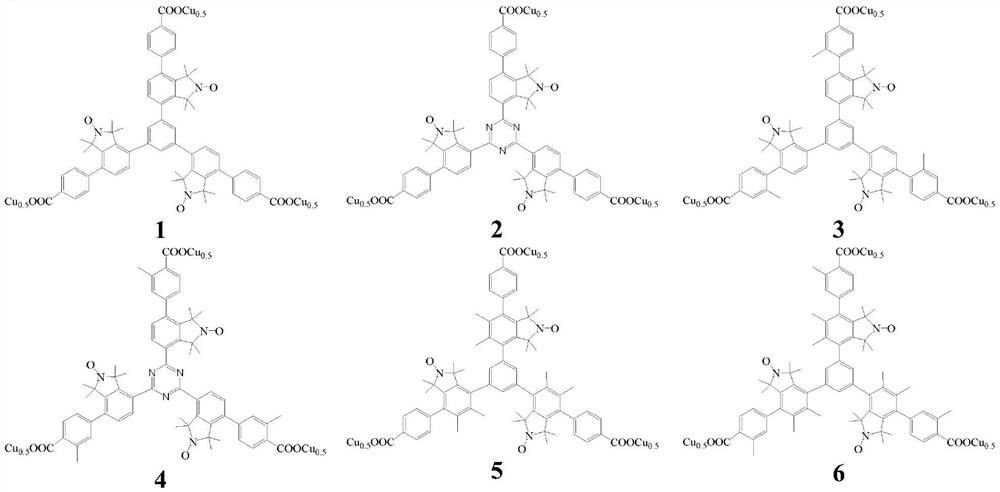
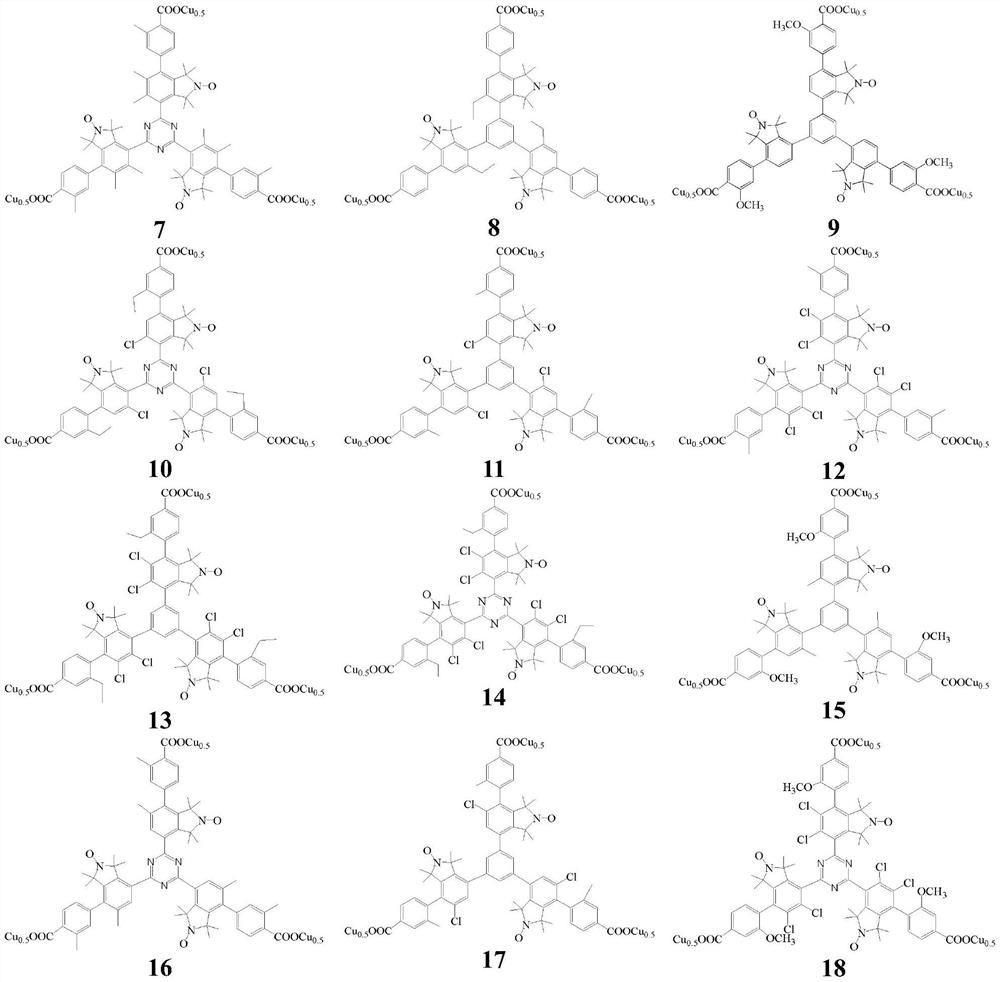
Copper complexes of triarylbenzene(triazine)tricarboxylates containing nitrogen and oxygen free radicals and their application in the preparation of menadione
A kind of technology of ternary aryl triazine tricarboxylate copper and tertiary aryl benzene tricarboxylate copper, which is applied in the field of application in the preparation of menaquinone by the nitrogen-oxygen free radical s-tertiary aryl benzene tricarboxylate copper complex. , which can solve the problems that metal salt catalyst cannot be recycled and reused, and the production cost is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (1) Preparation of nitrogen-oxygen free radical-s-triarylbenzene (triazine) bromide (20)
[0068] Under the protection of an inert gas (high-purity nitrogen), add 3.3 mmol of nitrogen-containing free radical dibromide (19), 1 mmol of triphenylboronic acid, 0.15 mmol of tetrakistriphenylphosphine palladium, and 6 mmol of carbonic acid to a 50 ml Schlek reaction tube. Potassium and 25ml of toluene water solvent (the volume ratio of toluene to water is 3:1), replace the reaction tube with nitrogen for 3 times, then heat to 90°C in an oil bath under magnetic stirring, and reflux for 24h; remove the oil bath, and react The solution was concentrated with a rotary evaporator, and the residue was separated with ethyl acetate as a developing solvent by thin-layer chromatography on silica gel to obtain a pure product containing nitrogen-oxygen radical-containing triarylbenzene (triazine) bromide (20), with a yield of 72%. . Mass spectrum (ESI) MS of the product: m / z: 877.1 (M+H)...
Embodiment 2
[0074] (1) Preparation of nitrogen-oxygen free radical-s-triarylbenzene (triazine) bromide (24)
[0075] Under the protection of an inert gas (high-purity nitrogen), add 4 mmol of nitrogen-containing free radical dibromide (23), 1 mmol of s-triazine boronic acid, 0.12 mmol of tetrakistriphenylphosphine palladium, and 6 mmol of cesium carbonate to a 50 ml Schlek reaction tube and 25ml of toluene water solvent (the volume ratio of toluene to water is 3:1), replace the reaction tube with nitrogen for 3 times, then heat to 90°C with an oil bath under magnetic stirring, and reflux the reaction for 20h, remove the oil bath, and the reaction solution Concentrate with a rotary evaporator, use ethyl acetate as a developing solvent for the residue, and separate with silica gel thin-layer chromatography to obtain a pure product containing nitrogen-oxygen radical-containing triarylbenzene (triazine) bromide (24), with a yield of 62%. Mass spectrum (ESI) MS of the product: m / z: 1069.8 (M-H...
Embodiment 3
[0081] (1) Preparation of nitroxy free radical-s-triarylbenzene ((triazine)) bromide (25)
[0082] The method is similar to Example 1, and the nitroxide-containing free radical-s-triarylbenzene ((triazine)) bromide (25) is prepared;
[0083] (2) Preparation of tertiary arylbenzene ((triazine)) tricarboxylic acid (26) containing nitrogen and oxygen free radicals
[0084] Under the protection of inert gas (high-purity nitrogen), in the Schlek reaction tube of 50ml, add the nitroxide-containing free radical s-triarylbenzene ((triazine)) bromide (25), 5mmol 4 that 1mmol step (1) makes - p-carboxyphenylboronic acid, 0.12mmol palladium chloride, 0.15mmol 2'-dicyclohexyl-2,6-dimethoxy-3-sulfonic acid-1,1'-biphenyl sodium salt, 6mmol sodium carbonate and 30ml Replace the reaction tube with nitrogen for 3 times, then heat to 100°C with an oil bath under magnetic stirring, and reflux for 32 hours; remove the oil bath, filter after the reaction, acidify the filtrate, adjust the pH to 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com