Separating method of isoflavone derivatives and application thereof
A separation method and technology of dimethoxyisoflavones, which are applied in the field of medicine and can solve problems such as activity detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Separation and structure confirmation of isoflavones
[0076] Red clover 2kg, with 8 times the amount of 75% ethanol as solvent, heated and refluxed for 3 extractions. Each time for 1.5 hours, filter and combine the filtrates, concentrate to obtain 200 g of ethanol extract; add water to suspend and dissolve, sequentially extract with petroleum ether and ethyl acetate, and extract 3 times respectively to obtain 45 g of ethyl acetate. Add appropriate amount of silica gel to the ethyl acetate fraction, mix the sample, perform silica gel column chromatography, and use gradient elution of dichloromethane-methanol system. The combined 9 fractions are recorded as Fr.1-Fr.9 according to the peak eluting time. Fr4 was chromatographed on a silica gel column, eluted with a dichloromethane-methanol gradient from 95:5 to 80:20, and colorless crystals were precipitated, which were designated as Fr4A. The structure was confirmed by NMR method, and its 1H-NMR spectral data ...
Embodiment 2
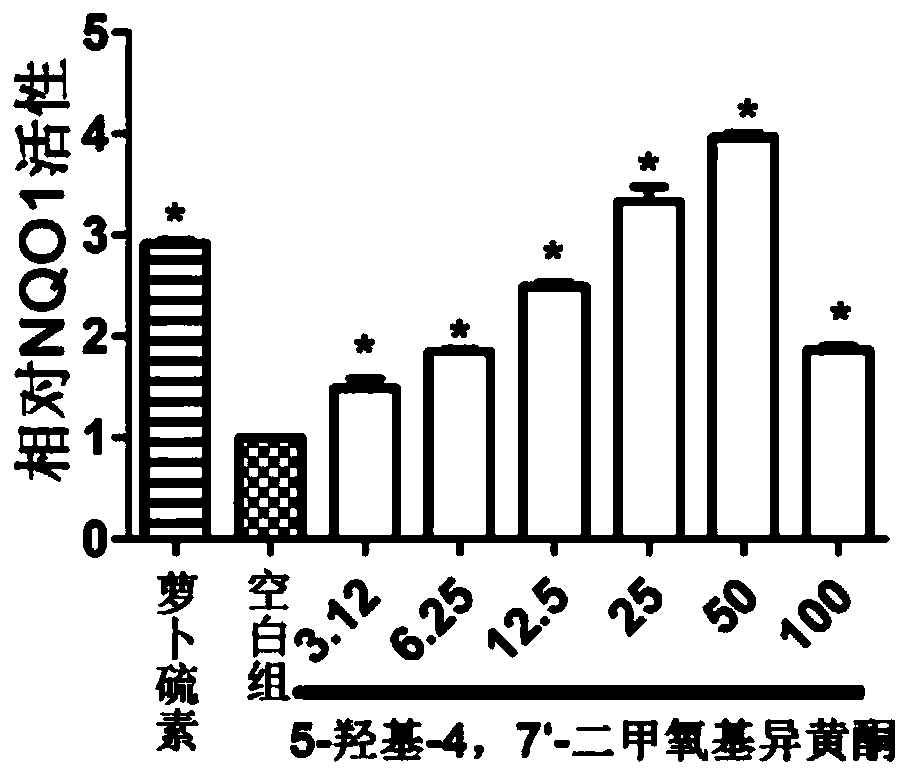
[0079] Example 2: Evaluation of NQO1-inducing activity of isoflavone 5-hydroxy-4,7'-dimethoxyisoflavone
[0080] (1) Culture of mouse hepatocarcinoma hepa 1c1c cell line
[0081] The mouse hepatocarcinoma hepa 1c1c cell line was purchased from the American Type Culture Collection (ATCC) and cultured in MEM medium containing 10% fetal bovine serum (FBS) in a 37°C, 5% CO2 incubator.
[0082] (2) NQO1-inducing activity test
[0083] The hepa 1c1c cells were inoculated on a 96-well plate, and different concentrations of isoflavones (confirmed in Example 1) were added after the cells adhered to the wall, treated for 24 hours, and the cells were lysed with 0.8% digitonin solution, and the detection solution (1.0mL 0.5M Tris-hydrochloric acid (Tris-HCl), 15 mg bovine serum albumin, 6 mg MTT, 150 μL Tween-20, 150 μL 150 mMD-glucose-6-phosphate, 15 μL 7.5 mM flavin adenine dinucleotide, 27 μL of 50 mM nicotinamide adenine dinucleotide phosphate, 20 μL of 50 mM menadione) were left fo...
Embodiment 3
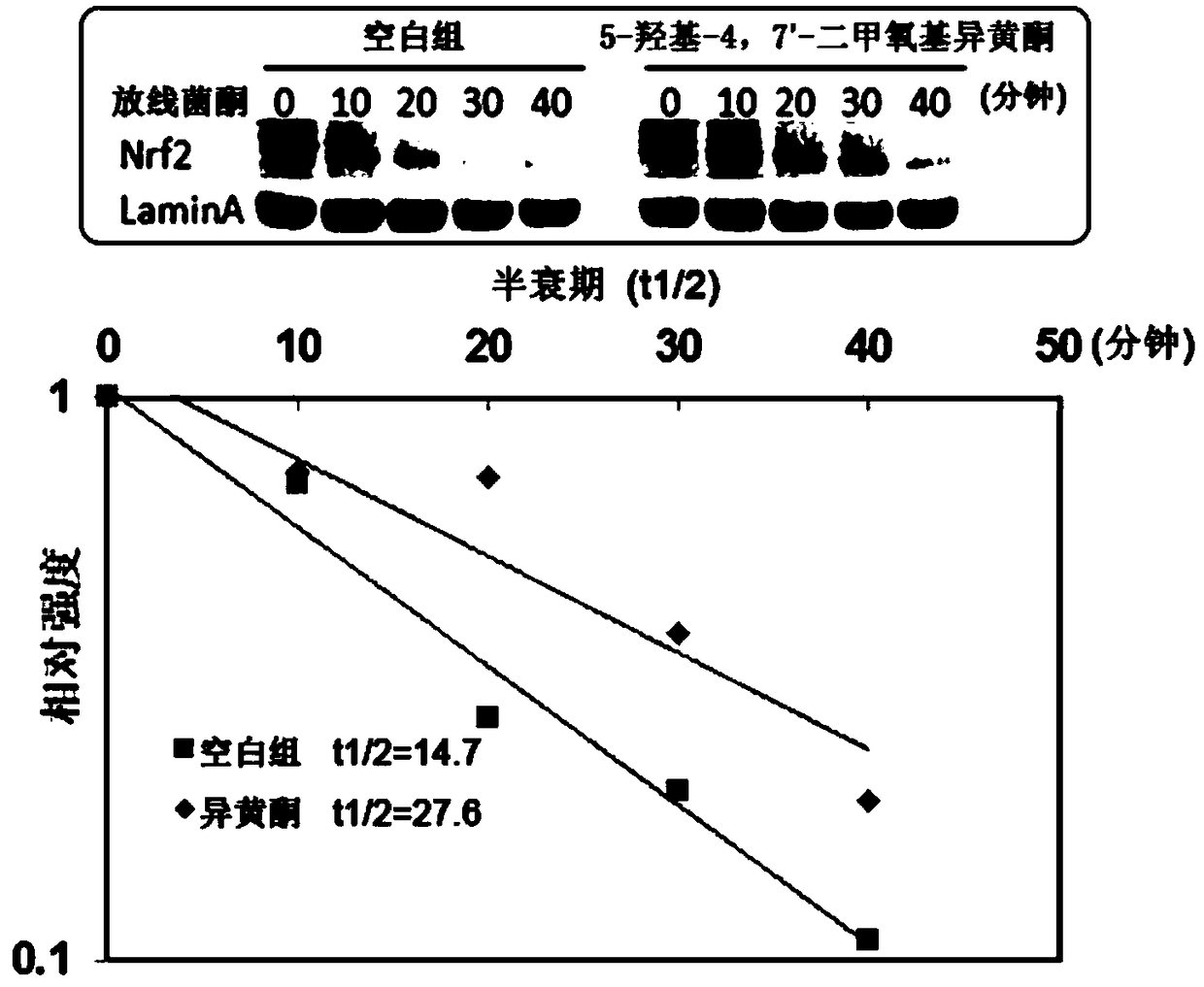
[0085] Embodiment 3: Isoflavone 5-hydroxyl-4,7'-dimethoxyisoflavone increases Nrf2 protein stability
[0086] (1) Culture of human normal epidermal Beas-2B cells
[0087] Human normal lung bronchial epithelial Beas-2B cells were purchased from the American Type Culture Collection (ATCC), using 1640 medium, and adding 10% fetal bovine serum (FBS) and 5% glutamine to it, and placed at 37°C , cultured in a 5% CO2 incubator.
[0088] (2) Western blot analysis to detect the half-life of Nrf2 protein
[0089] Inoculate Beas-2B cells in a 35mm diameter petri dish, culture until the density reaches 70%-80%, add isoflavones for 8 hours, then add cycloheximide, and time them at 0, 10, 20, 30, 40 minutes respectively Proteins were harvested, detected by Western blot analysis, and quantified using Image J software.
[0090] Result: if figure 2 As shown, the half-life of Nrf2 protein in Beas-2B cells in the blank group was 14.7 minutes, and after being treated with isoflavones for 8 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com