A-D-A type organic small molecule and preparation and application thereof
An A-D-A, small molecule technology, used in organic chemistry, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., can solve problems such as low solar cell efficiency, achieve high yield, and avoid the formation of unilateral by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
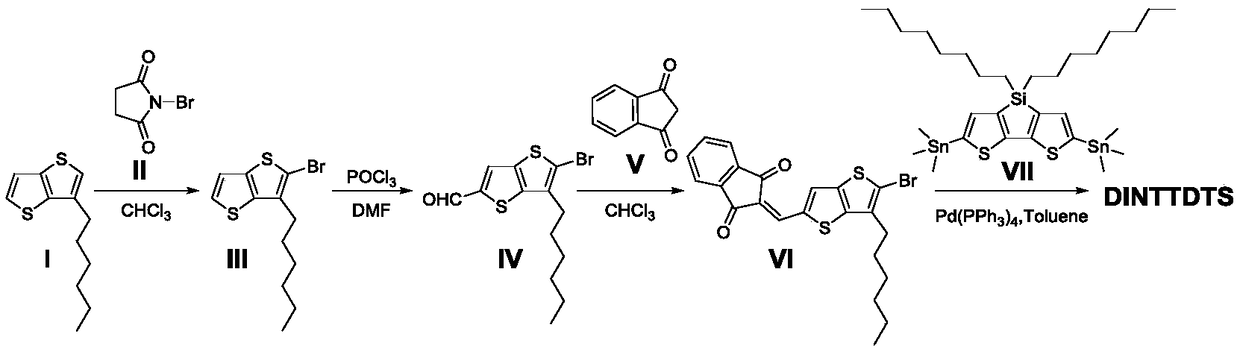
[0027] (1) Preparation of compound shown in formula (Ⅲ):
[0028] Under the protection of argon, add the compound (2.24g, 10.0mmol) represented by the formula (I) and 20mL of redistilled chloroform into a 100mL double-necked bottle, and add the compound represented by the formula (II) in batches under ice bath after the dissolution is complete. Compound (3.18g, 10.5mmol), stirred at room temperature (25-30°C) for 12h in the dark, poured the reaction solution into water (100mL) and extracted with dichloromethane (50mL×3), and the organic phase was washed with anhydrous MgSO 4 After drying, the filtrate was filtered to remove the solvent by a rotary evaporator to obtain a crude product. The crude product was passed through a silica gel chromatography column (the effective length of silica gel is 10 cm) with petroleum ether as the eluent, and the effluent fraction was collected to obtain the compound represented by formula (III) (2.83 g, 9.3 mmol, 93.5%). 1 H NMR (500MHz, CDCl ...
Embodiment 2
[0039]
[0040] (1) Preparation of compound shown in formula (IX):
[0041] Under the protection of argon, add the compound represented by formula (VII) (i.e., silacyclopentadithiophene with trimethyltin on both sides) (300mg, 0.403mmol) into a 100mL double-necked bottle pretreated in anhydrous and anaerobic conditions, The compound shown in formula (Ⅷ) (i.e. 2-isooctyl substituted thiophene bromaldehyde) (488.9mg, 1.612mmol) and ultra-dry toluene (30mL), after dissolution was complete, tetrakistriphenylphosphine palladium (186.0mg, 0.161 mmol), heated to 110°C and stirred at reflux for 24h. Cool to room temperature (25-30°C, the same below), pour the cooled reaction solution into water (100mL) and extract with dichloromethane (100mL×2), wash the organic phase twice with anhydrous MgSO 4 After drying, the filtrate was filtered to remove the solvent by a rotary evaporator to obtain a crude product. The crude product is passed through a silica gel chromatography column (the...
Embodiment 3
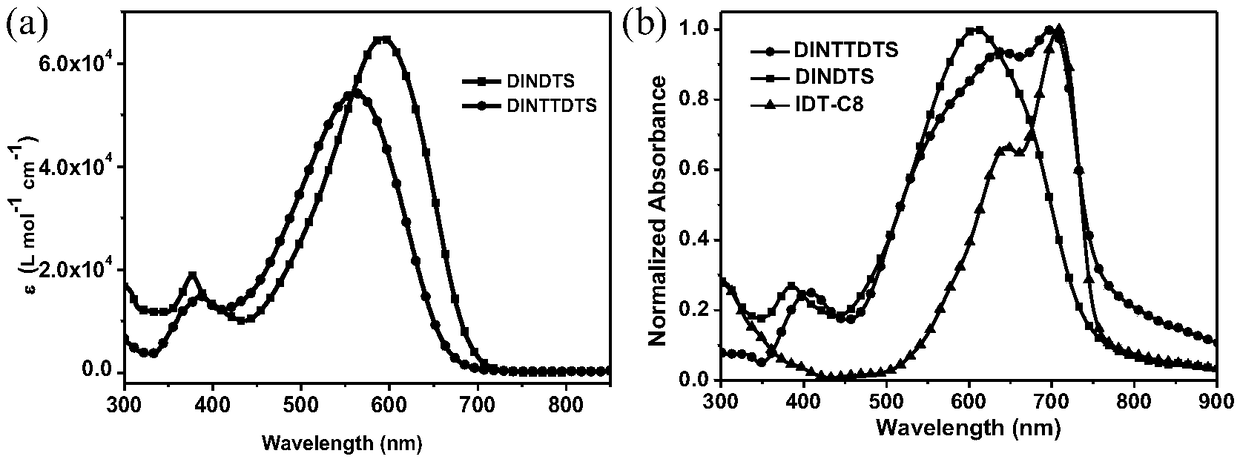
[0046] Embodiment 3 ultraviolet-visible absorption spectrum
[0047] Compound DINTTDTS in embodiment 2 is made into 10 respectively -5 mol / L and 10 -2 mol / L chloroform solution, respectively measure the UV absorption of the solution and the film, where the film is coated on a quartz plate with a spin coater at 1200rpm, the scanning range is 300-800nm, and the measuring instrument is Jasco V-570 UV / VIS / NIR Spectrophotometer and compared with compound DINDTS. The UV-Vis absorption curves of compounds DINDTS and DINTTDTS in chloroform are as follows figure 2 As shown in a, the maximum absorption peaks are 590nm and 561nm respectively, and the absorption coefficients are 6.49×10 4 m -1 cm -1 and 5.42×10 4 m -1 cm -1 . Compared with the compound DINDTS, the maximum absorption peak of the compound DINTTDTS with a longer effective conjugation degree has a blue shift. The LUMO energy level is mainly determined by the electron-withdrawing ability of the electron-deficient u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com