Trace iron (III) ion-doped nickel disulfide catalyst for oxygen evolution reaction of electrolysis water
A technology of trinickel disulfide and oxygen evolution reaction, which is applied in the direction of electrolysis process, electrolysis components, electrolysis inorganic material coating, etc., to achieve the effect of less iron doping, low cost and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Mix the following raw materials evenly, add hydrochloric acid to control the pH value of the system to 4, and prepare the electrodeposition solution:
[0031]
[0032] 2. Put nickel foam as the working electrode, platinum sheet as the counter electrode, and saturated calomel electrode as the reference electrode, put it into the electrodeposition solution prepared in step 1, and conduct electrodeposition by cyclic voltammetry. The scanning range is -0.8V~ -0.2V, the scan rate is 20mV / s, and the number of cycles is 10 times, and the iron (III) ion-doped trinickel disulfide catalyst is directly deposited on the nickel foam.
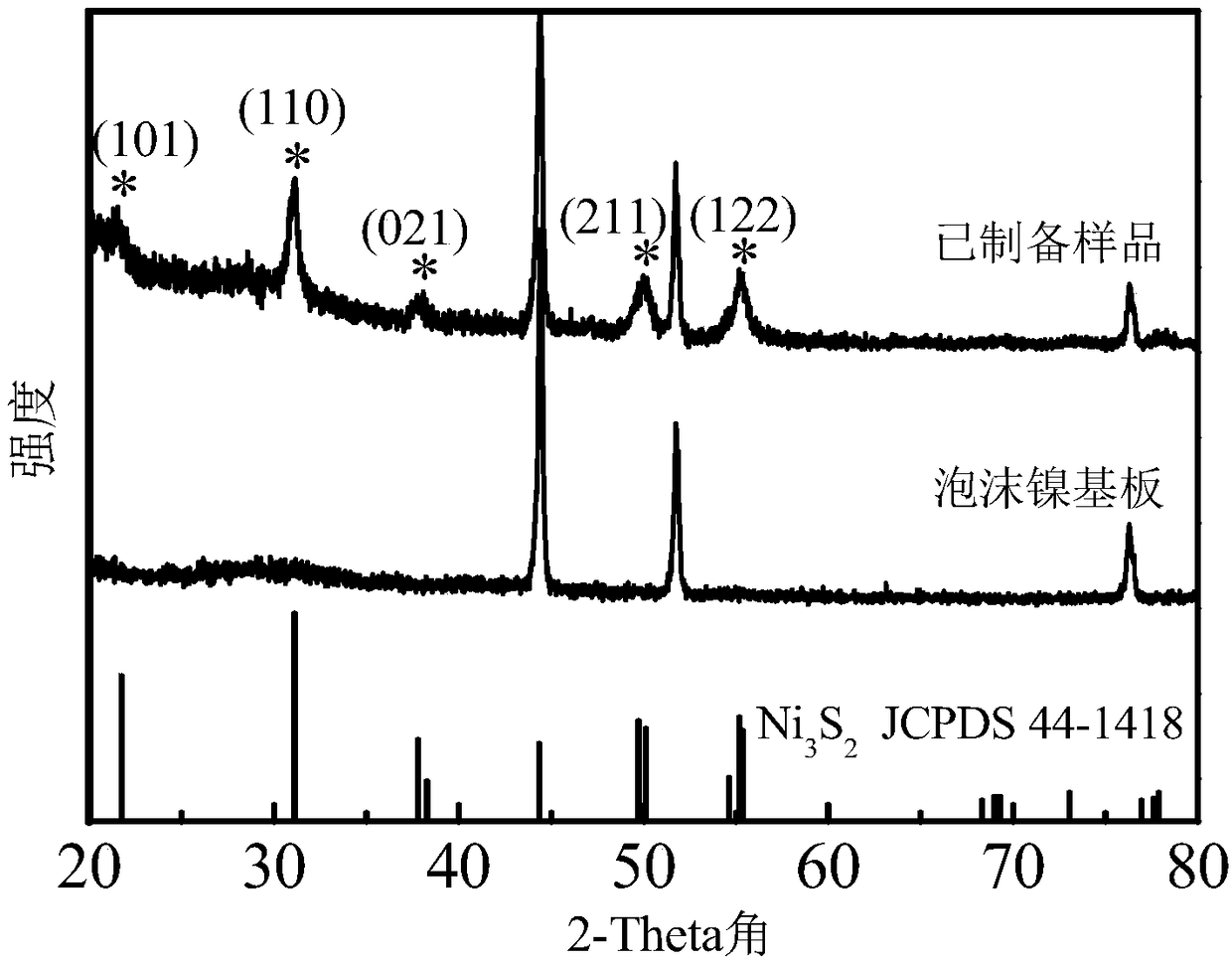
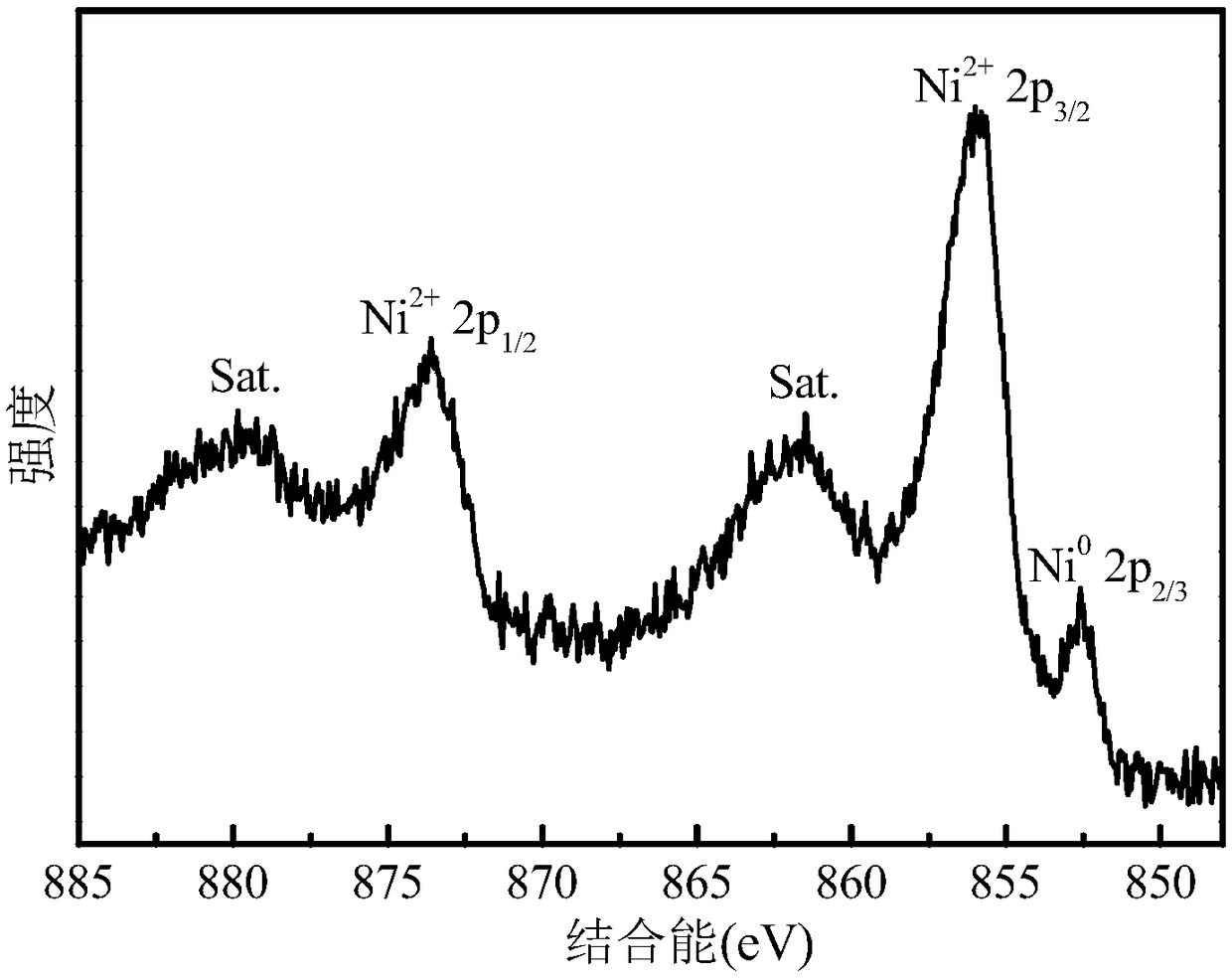
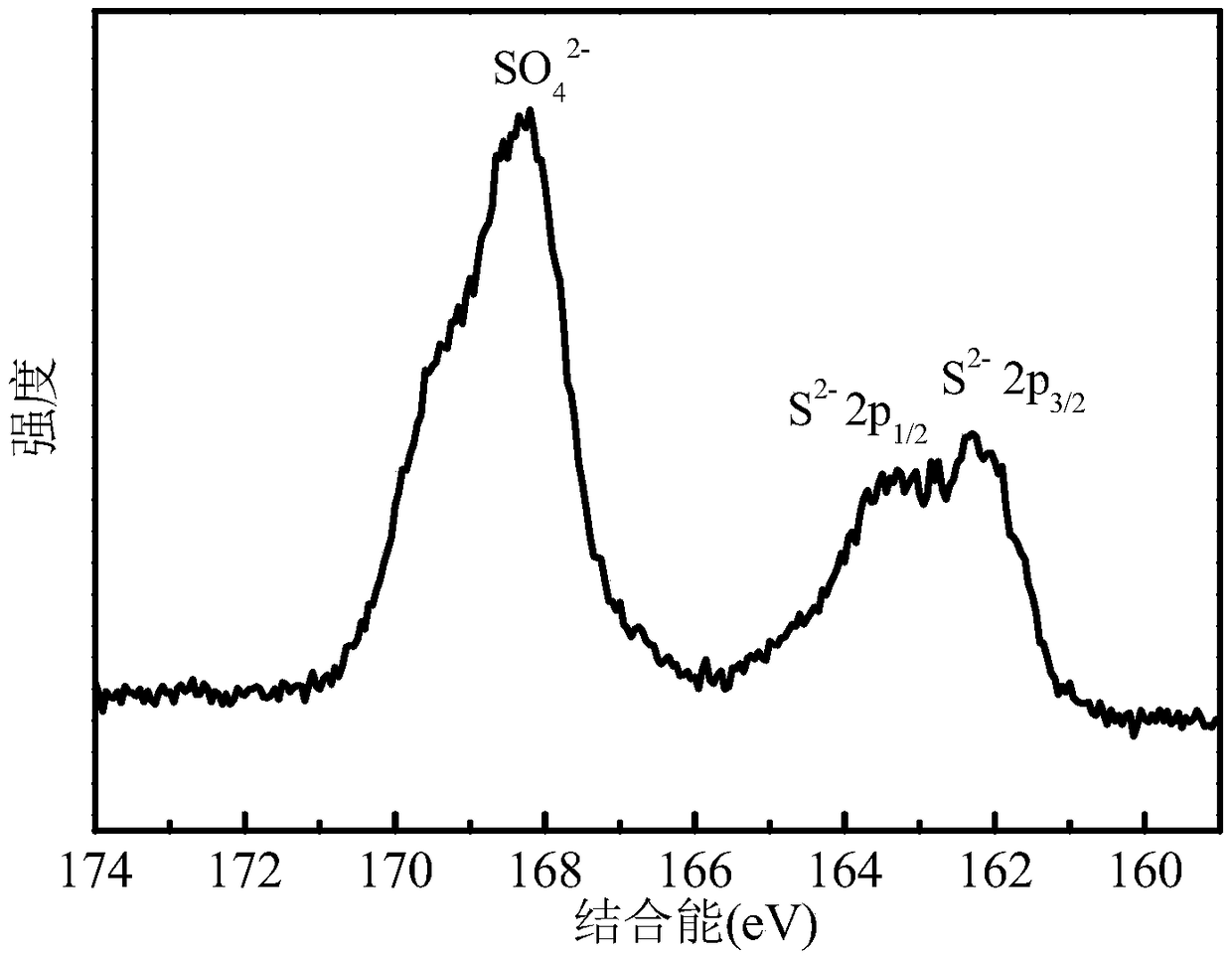
[0033] Depend on figure 1 It can be seen that the catalyst has five diffraction peaks marked with asterisks at 21.7°, 31.1°, 37.8°, 49.7° and 55.2°, corresponding to Ni 3 S 2 (101), (110), (003), (113) and (122) crystal planes (JCPDS no.44-1418), indicating the presence of crystalline Ni in the catalyst 3 S 2 Mutually. In addition, the cata...
Embodiment 2
[0035] In the present embodiment, the addition of ferrous chloride is increased to 2g, and other steps are identical with embodiment 1, obtain the trinickel disulfide catalyst (see Figure 6 ).
Embodiment 3
[0037] 1. Mix the following raw materials evenly, add hydrochloric acid to control the pH value of the system to 3.5, and prepare the electrodeposition solution:
[0038]
[0039]
[0040] 2. Put nickel foam as the working electrode, platinum sheet as the counter electrode, and saturated calomel electrode as the reference electrode, put it into the electrodeposition solution prepared in step 1, and conduct electrodeposition by cyclic voltammetry. The scanning range is -0.8V~ -0.2V, the scan rate is 10mV / s, and the number of cycles is 30 times, and the nickel disulfide catalyst doped with iron (III) ions is directly deposited on the foamed nickel (see Figure 7 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com