Ultraviolet light-triggered crosslinking near-infrared molecular probe as well as preparation method and application thereof
A molecular probe, near-infrared technology, applied in chemical instruments and methods, medical preparations containing active ingredients, pharmaceutical formulations, etc. Mild triggering conditions and efficient cross-linking reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
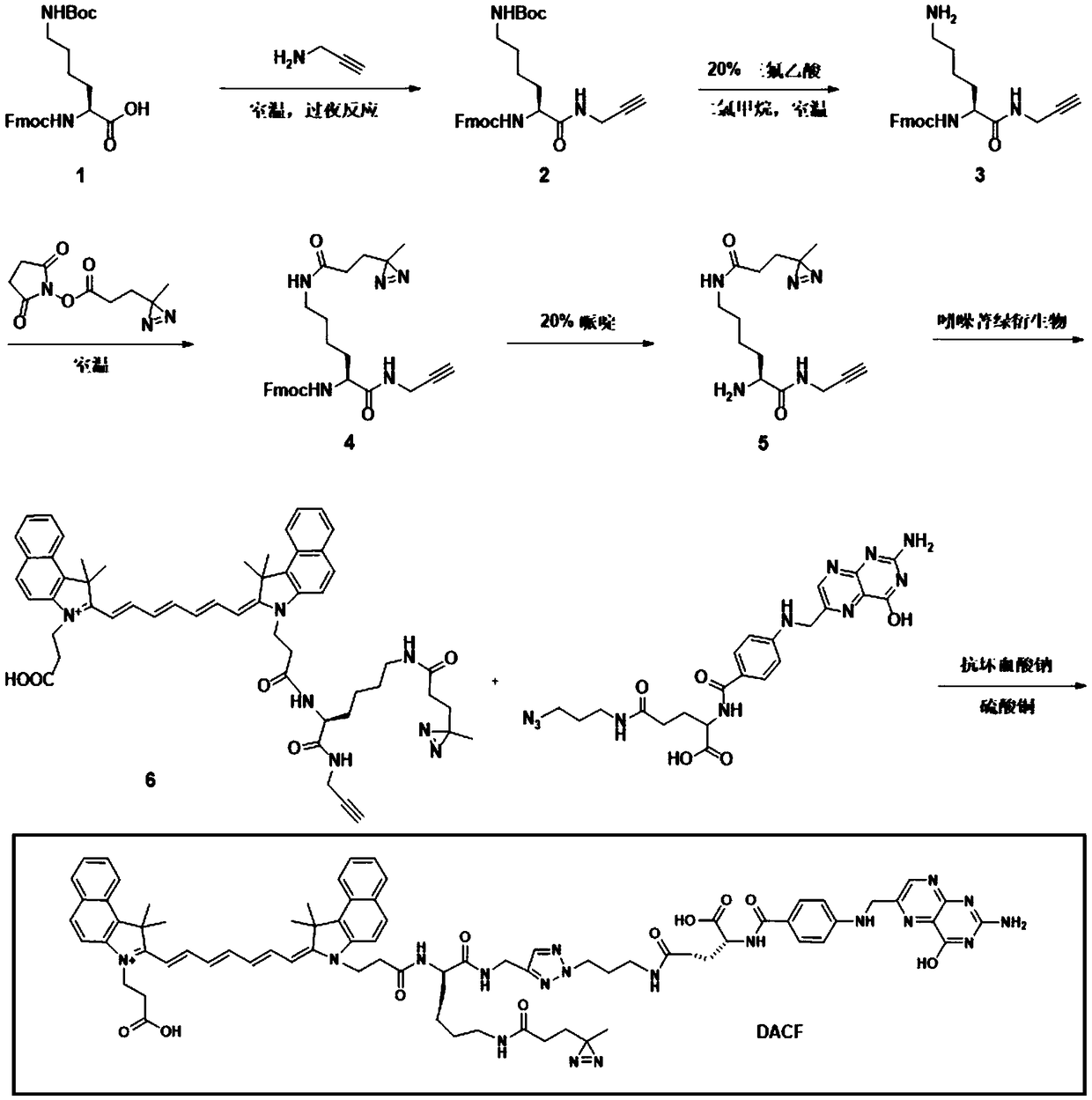
[0069] Example 1: Synthesis and characterization of photocrosslinkable near-infrared molecular probes
[0070] (1) Add propargylamine (0.29 mL), N,N-dimethylformamide (30 mL), HOBt (0.68 g, 5.04 mmol), HBTU (1.91 g, 5.04 mmol) and Diisopropylethylamine (1.08 g, 8.4 mmol) was stirred evenly. Subsequently, N-tert-butoxycarbonyl-N'-fluorenylmethoxycarbonyl-D-lysine (1.97 g, 4.2 mmol) was added into the reaction flask, stirring was continued, and the reaction was carried out at room temperature overnight (20 hours). After the reaction, the solvent was removed by rotary evaporation, and 30 mL of ethyl acetate was added to redissolve the intermediate compound. Then the organic phase was washed once with 30 mL deionized water, saturated sodium bicarbonate and sodium chloride aqueous solution respectively, dried with anhydrous magnesium sulfate and then spin-dried to obtain white powdery intermediate 1 (its structure is as follows: figure 1 Compound 1 in ) (1.95 g, yield: 92%).
[...
Embodiment 2
[0082] Example 2: Photocrosslinking of photocrosslinkable near-infrared molecular probe DACF after ultraviolet light irradiation
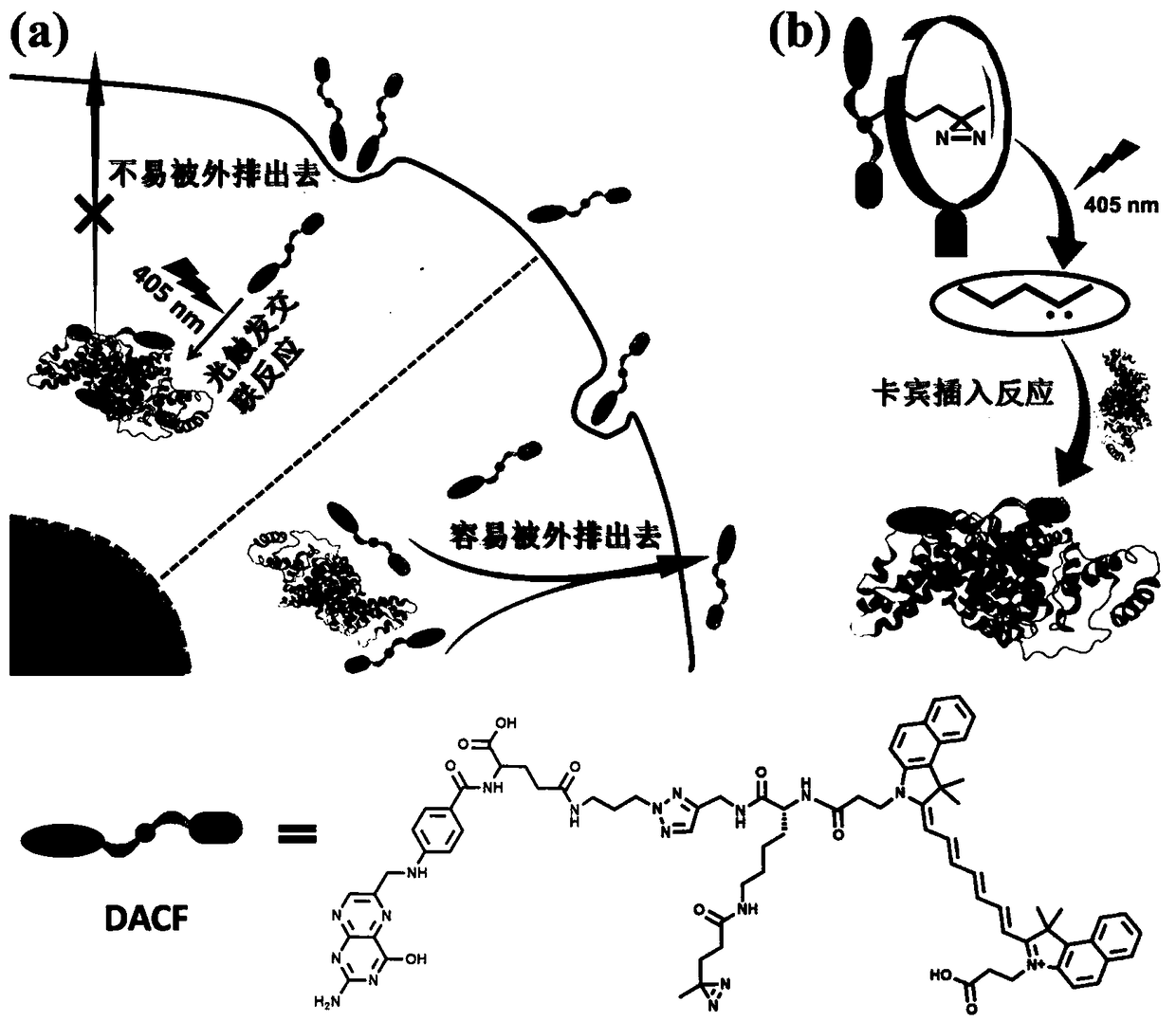
[0083] Such as figure 2 As shown, the photocrosslinkable near-infrared molecular probe DACF prepared in Example 1 was diluted into the cell culture medium, and then added to the 4T1 cell culture dish for co-incubation. When the probe enters the tumor cell, it is irradiated with ultraviolet light, and the group carried by the probe will generate an intermediate carbene, which will then be covalently bonded to the surrounding macromolecular proteins through C-H, O-H, N-H and S-H insertion reactions, making the probe The needle molecule is firmly connected with the macromolecular protein in the tumor cell, thereby prolonging the residence time of the probe molecule in the cell and improving the imaging and therapeutic effect of the probe molecule. For the mechanism, please refer to the attached figure 2 .
Embodiment 3
[0084] Example 3: HPLC purity characterization and high-resolution mass spectrometry characterization of photocross-linked near-infrared molecular probe DACF
[0085] After diluting the photocross-linked near-infrared molecular probe prepared in Example 1 to a concentration of 5 μM with solvent methanol, the molecular weight of the probe was determined by high-resolution mass spectrometry, and its purity was determined by high-performance liquid chromatography. analyze.
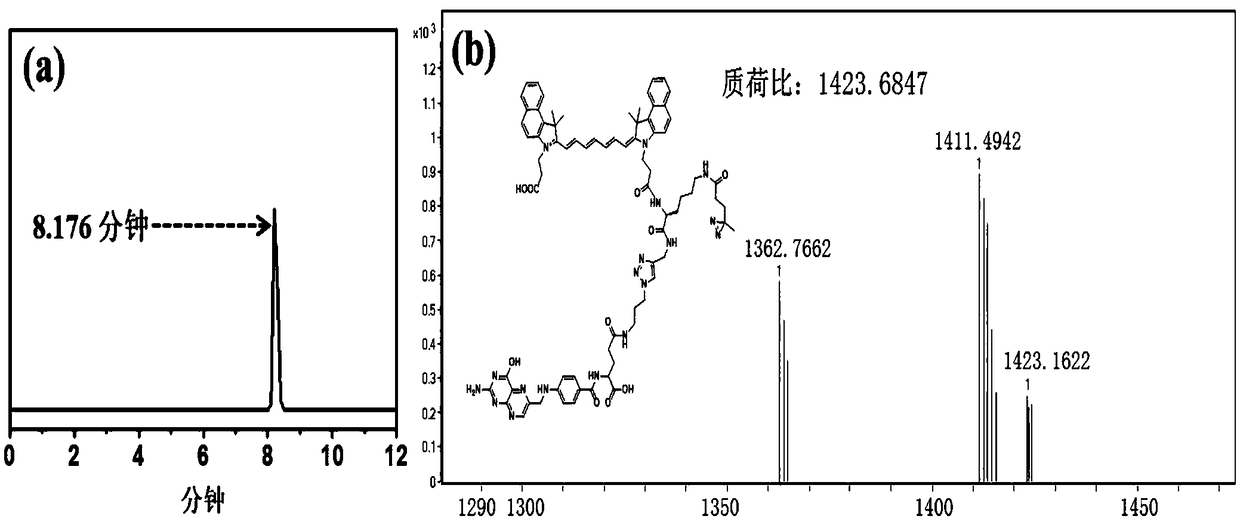
[0086] Such as image 3 As shown in a, the sample was analyzed by Agilent 1260 high-performance liquid chromatography, and the retention time of the probe DACF was 8.176 minutes. After further integrating the peak area, the concentration of the probe in the sample was calculated as high as 99%. image 3 b shows the theoretical m / z of the probe DACF: 1423.6847, and the m / z in the high-resolution mass spectrum actually obtained: 1423.1622, the two are consistent, which is the desired compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com