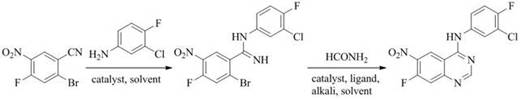
A new method for preparing n-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitro-4-quinazolinamine
A technology of quinazoline amine and fluorophenyl, which is applied in the field of organic chemical manufacturing technology, and can solve problems such as difficult availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of 2-bromo-N-(3-chloro-4-fluorophenyl)-4-fluoro-5-nitrobenzamidine
[0025]In a special microwave reaction flask equipped with a drying tube and a condenser, add 2-bromo-4-fluoro-5-nitrobenzonitrile (2.44 g, 10 mmol), 3-chloro-4-fluoroaniline (1.60 g , 11 mmol), cuprous chloride (0.30 g, 3 mmol), absolute ethanol (20 mL), and reflux for 30 min under microwave irradiation. Cool the reaction solution to room temperature, filter with suction, distill off ethanol under reduced pressure, filter with suction, wash the solid with water, and dry to obtain 2-bromo-N-(3-chloro-4-fluorophenyl)-4-fluoro as a pale yellow solid - 5-nitrobenzamidine (2.96 g, 76%).
Embodiment 2
[0026] Example 2: Preparation of 2-bromo-N-(3-chloro-4-fluorophenyl)-4-fluoro-5-nitrobenzamidine
[0027] In a special microwave reaction flask equipped with a drying tube and a condenser, add 2-bromo-4-fluoro-5-nitrobenzonitrile (2.44 g, 10 mmol), 3-chloro-4-fluoroaniline (1.45 g , 10 mmol), cuprous bromide (0.14 g, 1 mmol), glacial acetic acid (30 mL), and reflux for 25 min under microwave irradiation. The reaction solution was cooled to room temperature, suction filtered, acetic acid was distilled off under reduced pressure, suction filtered, the solid was washed with water, and dried to obtain a pale yellow solid 2-bromo-N-(3-chloro-4-fluorophenyl)-4-fluoro - 5-nitrobenzamidine (3.17 g, 81%).
Embodiment 3
[0028] Example 3: Preparation of 2-bromo-N-(3-chloro-4-fluorophenyl)-4-fluoro-5-nitrobenzamidine
[0029] In a special microwave reaction flask equipped with a drying tube and a condenser, add 2-bromo-4-fluoro-5-nitrobenzonitrile (2.44 g, 10 mmol), 3-chloro-4-fluoroaniline (1.45 g , 10 mmol), cuprous iodide (0.38 g, 2 mmol), absolute ethanol (10 mL), and reflux under microwave irradiation for 20 min. Cool the reaction solution to room temperature, filter with suction, distill off ethanol under reduced pressure, filter with suction, wash the solid with water, and dry to obtain 2-bromo-N-(3-chloro-4-fluorophenyl)-4-fluoro as a pale yellow solid - 5-nitrobenzamidine (3.66 g, 94%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com