Albendazole liposome and preparation process thereof
A technology of albendazole liposome and albendazole is applied in the field of albendazole liposome and its preparation technology, and can solve the problem of low absorption rate, low intestinal absorption rate and bioavailability, slow absorption process, etc. problems, to achieve the effect of increasing targeting and pertinence, increasing bioavailability, and accelerating the absorption process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
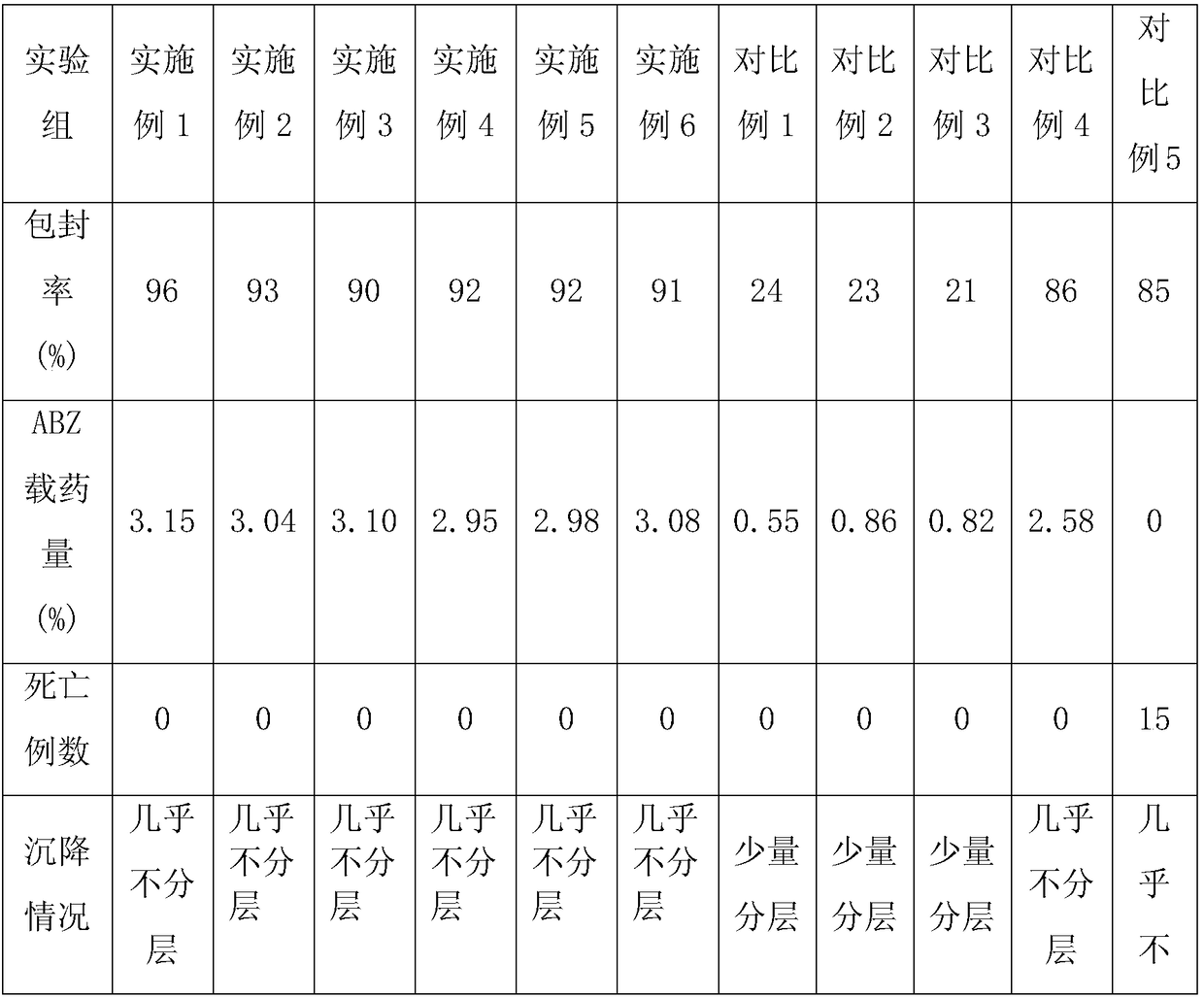
Embodiment 1
[0028] A preparation method for albendazole liposomes, comprising the following steps:
[0029] A, get 10 parts by weight of citric acid and the sodium citrate of 8 parts by weight, add water to dissolve and prepare the citric acid buffer solution that concentration is 18g / L;
[0030] b. 12 parts by weight of albendazole are added to the mixed solution of polyethylene glycol, stirred and dissolved to obtain the albendazole solution;
[0031] c, 30 parts by weight of 1-palmitoyl-2-oleoylphosphatidylglycerol, 10 parts by weight of dipalmitoylphosphatidylserine, 15 parts by weight of dierucoylphosphatidylethanolamine, and 8 parts by weight of pyrantel are added to Add absolute ethanol to the beaker, stir and dissolve in a water bath at 70°C for 40 minutes and volatilize the ethanol to form a film, add the citric acid buffer solution prepared in step a, stir, and hydrate at 70°C for 40 minutes to obtain a liposome solution;
[0032] d, passing the liposome solution prepared in st...
Embodiment 2
[0035] A preparation method for albendazole liposomes, comprising the following steps:
[0036] a, get 10 parts by weight of citric acid and 7 parts by weight of sodium citrate, add water to dissolve and prepare a citric acid buffer solution with a concentration of 17g / L;
[0037] b. 12 parts by weight of albendazole are added to the mixed solution of polyethylene glycol, stirred and dissolved to obtain the albendazole solution;
[0038] c, 30 parts by weight of 1-palmitoyl-2-oleoylphosphatidylglycerol, 8 parts by weight of dipalmitoylphosphatidylserine, 15 parts by weight of dierucoylphosphatidylethanolamine, and 8 parts by weight of pyrantel are added to Add absolute ethanol to the beaker, stir and dissolve in a water bath at 65°C for 35 minutes and volatilize the ethanol to form a film, add the citric acid buffer solution prepared in step a, stir, and hydrate at 65°C for 35 minutes to obtain a liposome solution;
[0039] d, passing the liposome solution prepared in step c ...
Embodiment 3
[0042] A preparation method for albendazole liposomes, comprising the following steps:
[0043] A, get 9 parts by weight of citric acid and the sodium citrate of 8 parts by weight, add water to dissolve and prepare the citric acid buffer solution that concentration is 18g / L;
[0044] b. 10 parts by weight of albendazole are added to the mixed solution of polyethylene glycol, stirred and dissolved to obtain the albendazole solution;
[0045] c, 33 parts by weight of 1-palmitoyl-2-oleoylphosphatidylglycerol, 10 parts by weight of dipalmitoylphosphatidylserine, 16 parts by weight of dierucoylphosphatidylethanolamine, and 8 parts by weight of pyrantel are added to Add absolute ethanol to the beaker, stir and dissolve in a water bath at 70°C for 40 minutes and volatilize the ethanol to form a film, add the citric acid buffer solution prepared in step a, stir, and hydrate at 70°C for 39 minutes to obtain a liposome solution;
[0046] d, passing the liposome solution prepared in step ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com